
The authors test the usefulness of zinc oxide microparticles relative to zinc oxide nanoparticles as antibacterial agents.
Read More...Surface cleanliness of hydrothermally grown zinc oxide microparticles compared to commercial nanoparticles

The authors test the usefulness of zinc oxide microparticles relative to zinc oxide nanoparticles as antibacterial agents.
Read More...How are genetically modified foods discussed on TikTok? An analysis of #GMOFOODS

Here, the authors investigated engagement with #GMOFOODS, a hashtag on TikTok. They hypothesized that content focused on the negative effects of genetically modified organisms would receive more interaction driven by consumers. They found that the most common cateogry focused on the disadvantages of GMOs related to nutrition and health with the number of views determining if the video would be provided to users.
Read More...Impact of NaCl concentration in crystalline nanocellulose for printed ionic dielectrics
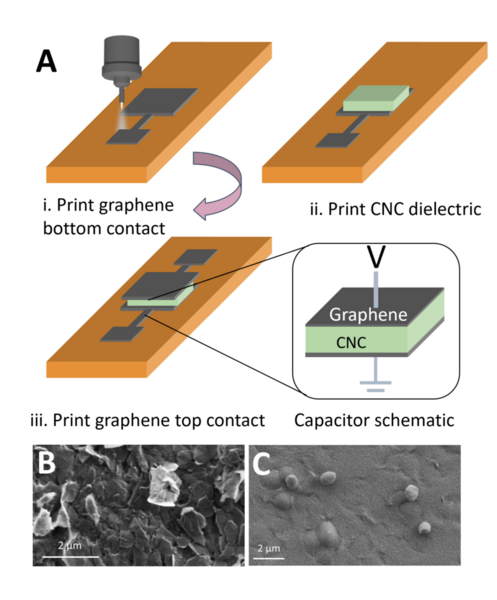
The authors looked at how the addition of NaCl to crystalline nanocellulose capacitors could improve performance in transistor applications. They found that NaCl can improve performance, but that further work is needed to determine the optimal concentration used depending on the intended application.
Read More...The effect of an anthocyanin on the gut permeability of a Type 2 Diabetic Drosophila melanogaster

Anti-diabetic drugs like Metformin are known to increase gut permeability, and this has a negative impact on patient health. These authors hypothesized that this can be mitigated using purple sweet potato extract, which is high anthocyanin content, that feeds bacteria metabolism to decrease gut permeability.
Read More...Innovative Treatment for Reducing Senescence and Revitalizing Aging Cells through Gene Silencing
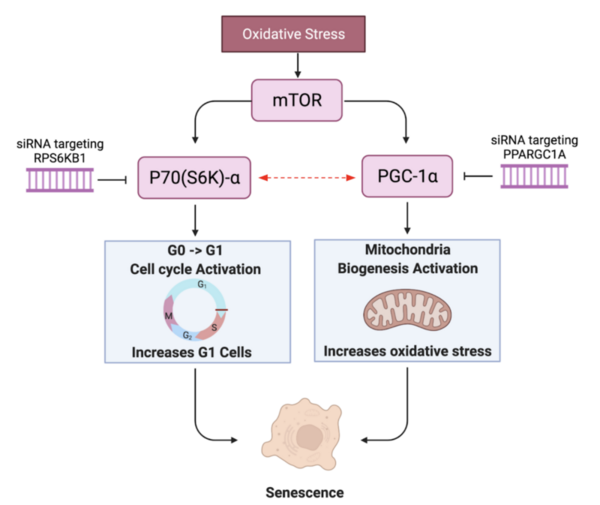
Cellular senescence plays a key role in aging cells and is attributed to a number of disease and pathology. These authors find that genetic editing of both RPS6KB1 and PPARGC1A revitalizes a human skin fibroblast cell line.
Read More...Anti-inflammatory and pro-apoptotic properties of the polyherbal drug, MAT20, in MCF-7 cells
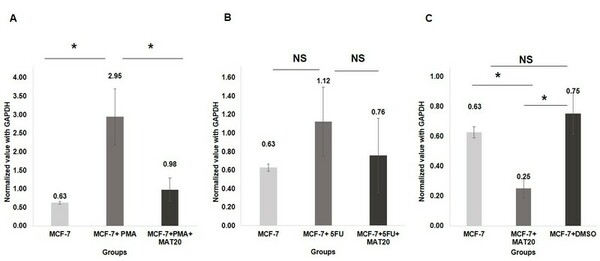
The authors test potential anti-inflammatory and pro-apoptotic effects of a polyherbal extract formulation on cultured breast cancer cells.
Read More...Integrating microbial fuel cell with sedum green roof for stormwater retention and renewable energy generation
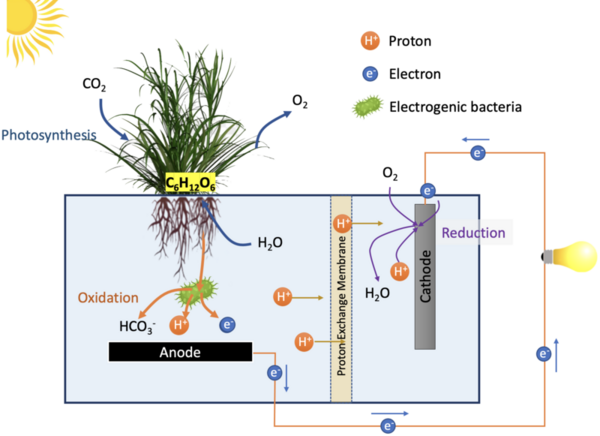
The authors looked at renewable energy generators and the ability to utilize green roofs as a solution to climate change.
Read More...Changing public opinions on genetically modified organisms through access to educational resources

Genetically modified organisms (GMOs) are crops or animals that have been genetically engineered to express a certain physical or biological characteristic and have various benefits that have made them become increasingly popular. However, the public has had mixed reactions to the use of GMOs, with some skeptical of their safety. The purpose of this study was to evaluate how opinions on genetically modified foods can change from exposure to small amounts of information
Read More...Optimizing 3D printing parameters: Evaluating infill type and layer height effects on tensile fracture force
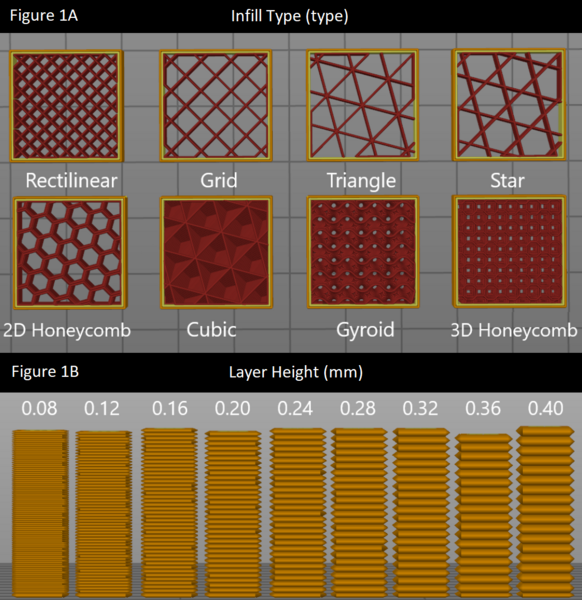
In this study, the authors test different infill patterns to determine which would be the strongest and most durable for 3D printing applications, which have become an integral part of many facets of life.
Read More...High-throughput virtual screening of novel dihydropyrimidine monastrol analogs reveals robust structure-activity relationship to kinesin Eg5 binding thermodynamics
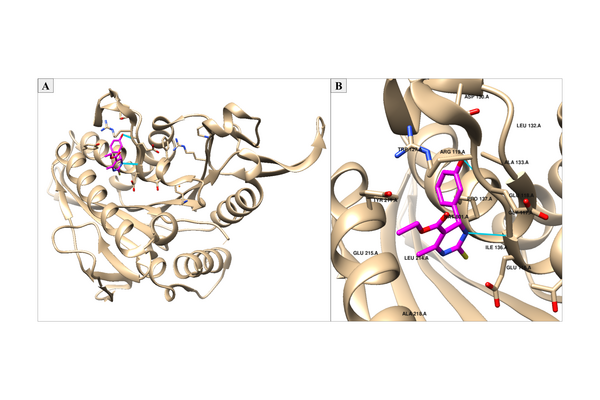
As cancer continues to take millions of lives worldwide, the need to create effective therapeutics for the disease persists. The kinesin Eg5 assembly motor protein is a promising target for cancer therapeutics as inhibition of this protein leads to cell cycle arrest. Monastrol, a small dihydropyrimidine-based molecule capable of inhibiting the kinesin Eg5 function, has attracted the attention of medicinal chemists with its potency, affinity, and specificity to the highly targeted loop5/α2/α3 allosteric binding pocket. In this work, we employed high-throughput virtual screening (HTVS) to identify potential small molecule Eg5 inhibitors from a designed set of novel dihydropyrimidine analogs structurally similar to monastrol.
Read More...Search articles by title, author name, or tags