High-throughput virtual screening of novel dihydropyrimidine monastrol analogs reveals robust structure-activity relationship to kinesin Eg5 binding thermodynamics
(1) Mission San Jose High School, Fremont, CA, (2) Dougherty Valley High School, San Ramon, CA, (3) Dublin High School, Dublin, CA , (4) Department of Chemistry, Biochemistry & Physics, Aspiring Scholars Directed Research Program, Fremont, CA
https://doi.org/10.59720/20-060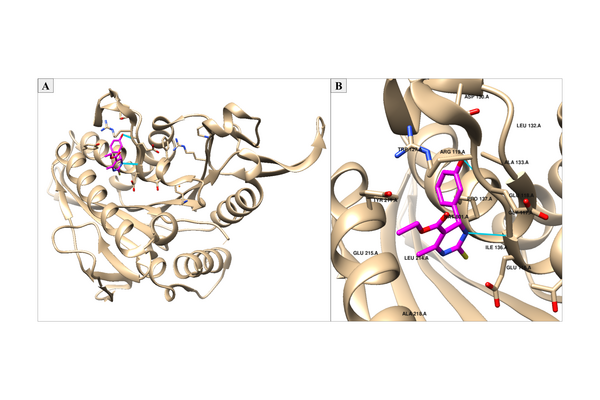
As cancer continues to take millions of lives worldwide, the need to create effective therapeutics for the disease persists. The kinesin Eg5 assembly motor protein is a promising target for cancer therapeutics as inhibition of this protein leads to cell cycle arrest. Monastrol, a small dihydropyrimidine-based molecule capable of inhibiting the kinesin Eg5 function, has attracted the attention of medicinal chemists with its potency, affinity, and specificity to the highly targeted loop5/α2/α3 allosteric binding pocket. In this work, we employed high-throughput virtual screening (HTVS) to identify potential small molecule Eg5 inhibitors from a designed set of novel dihydropyrimidine analogs structurally similar to monastrol. Density functional theory (DFT) calculations and protein-ligand docking experiments revealed that the analogs with geranyl ester substitutions exhibited the greatest binding affinities to the allosteric binding pocket of kinesin Eg5. In-depth analysis of the binding pocket amino acid residues and calculations of the cLogP value for each compound demonstrated qualitatively and quantitatively that strong hydrophobic interactions of the ester functionality with kinesin Eg5 are of great significance in the improved binding of dihydropyrimidine analogs. This establishment of a quantitative structure-activity relationship to kinesin Eg5 binding thermodynamics using HTVS revealed the discovery of improved dihydropyrimidine-based inhibitors capable of advancing society’s progress in the fight against cancer.
This article has been tagged with: