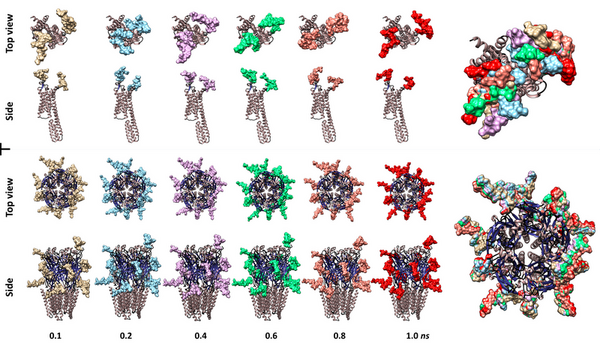
Here, seeking to better understand the roles of glycans in the receptors of active sites of neuronal cells, the authors used molecular dynamics simulations to to uncover the dynamic nature of N-glycans on membrane proteins. The authors suggest the study of theinteractions of these membrane poreins could provide future potential therapeutic targets to treat mental diseases.
Read More...