Computational Structure-Activity Relationship (SAR) of Berberine Analogs in Double-Stranded and G-Quadruplex DNA Binding Reveals Both Position and Target Dependence
(1) BASIS Independent Silicon Valley, San Jose, California, (2) Amador Valley High School, Pleasanton, California, (3) Foothill High School, Pleasanton, California, (4) Mission San Jose High School, Fremont, California, (5) Los Altos High School, Los Altos, California, (6) The College Preparatory School, Oakland, California, (7) Department of Chemistry, Biochemistry, & Physical Science, Fremont, California
https://doi.org/10.59720/20-097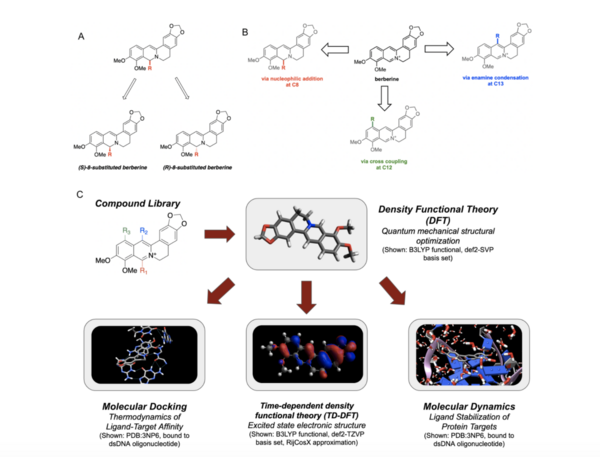
Berberine, a natural product alkaloid, and its analogs have a wide range of medicinal properties, including antibacterial and anticancer effects. Previous studies showed that berberine and its analogs intercalate into DNA, thereby inhibiting DNA replication. Berberine has also been studied as a photosensitizer and was shown to generate reactive singlet oxygen in situ, and this has applications in photodynamic therapy. Various groups have synthesized berberine analogs that have comparable or improved biological activity; however, an exhaustive structure-activity relationship of the free energy of binding of berberine analogs with substitution at C-8, C-12, and C-13 to DNA have not been previously reported. High throughput virtual screening (HTVS) allows for efficient analysis of compound libraries to identify lead compounds as possible pharmaceutical agents. Here, we employed HTVS towards a library of alkyl or aryl berberine analogs on C-8, C-12, and C-13 to probe binding to double-stranded and G-quadruplex DNA. Predicted free energies of binding to double-stranded DNA and G-quadruplex DNA were generated via molecular docking. The excited state electronic structure calculations were conducted via time-dependent density functional theory to probe the potential photosensitizing activity of each compound, and the potential G-quadruplex stabilizing abilities of key berberine analogs were probed through molecular dynamics simulations on a 4.0 nanosecond timescale. We determined that the nature of the substituent, the position of the substituent, and the nucleic acid target affect the free energy of binding of berberine analogs to DNA and G-quadruplex DNA, however berberine analogs did not result in net stabilization of G-quadruplex DNA.