
In this study, the authors address the concerns of heavy metal contamination in industrial and feedlot water waste. They test whether added probiotics are capable of taking up heavy metals in water to attenuate pollution.
Read More...Probiotic biosorption as a way to remove heavy metal in seawater

In this study, the authors address the concerns of heavy metal contamination in industrial and feedlot water waste. They test whether added probiotics are capable of taking up heavy metals in water to attenuate pollution.
Read More...Gene expression analysis of febrile seizure’s impact on mesial temporal lobe epilepsy
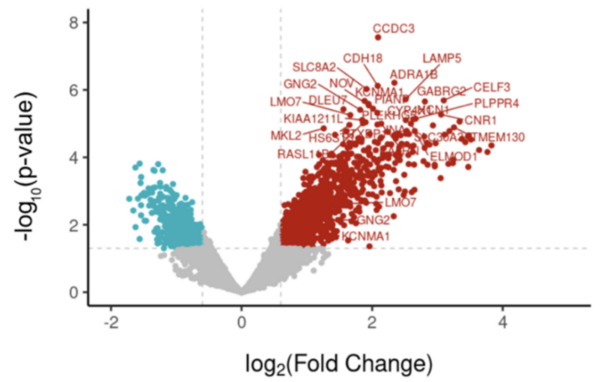
The authors looked at genes that were differentially expressed in patients who had and had not had febrile seizures to determine what differences in gene expression existed.
Read More...High-performance liquid chromatography insight in pH-dependent hydrolysis of andrographolide acetonide
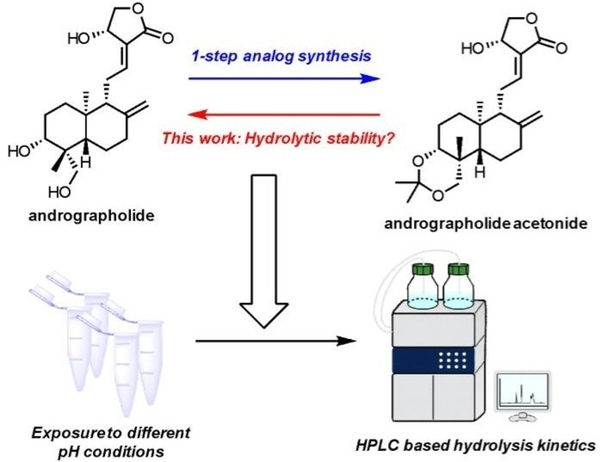
Andrographolide, a natural compound with anti-inflammatory, antidepressant, and anti-cancer properties, can be chemically modified by adding an acetonide group to form andrographolide acetonide, which is more potent and acts as a pH-dependent prodrug. Researchers investigated the hydrolysis of this acetonide group under mildly acidic conditions.
Read More...Sex differences in sleep disorders of Parkinson’s disease patients associated with a genetic risk variant
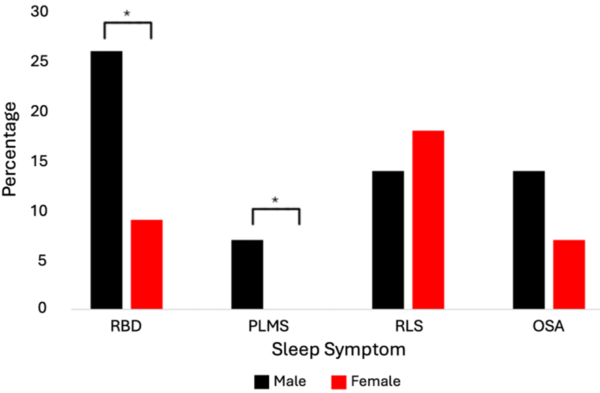
The authors use known Parkinson's disease-associated genetic variants to compare the prevalence of sleep dysfunction between males and females diagnosed with Parkinson's disease.
Read More...Synthesis of sodium alginate composite bioplastic films

The authors looked at the development of biodegradable bioplastic and its features compared to PET packaging films. They were able to develop a biodegradable plastic with sodium alginate that dissolved in water and degrade in microbial conditions while also being transparent and flexible similar to current plastic films.
Read More...How are genetically modified foods discussed on TikTok? An analysis of #GMOFOODS

Here, the authors investigated engagement with #GMOFOODS, a hashtag on TikTok. They hypothesized that content focused on the negative effects of genetically modified organisms would receive more interaction driven by consumers. They found that the most common cateogry focused on the disadvantages of GMOs related to nutrition and health with the number of views determining if the video would be provided to users.
Read More...Applying centrality analysis on a protein interaction network to predict colorectal cancer driver genes
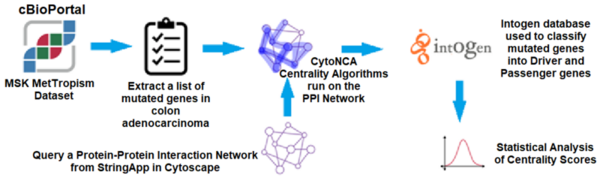
In this article the authors created an interaction map of proteins involved in colorectal cancer to look for driver vs. non-driver genes. That is they wanted to see if they could determine what genes are more likely to drive the development and progression in colorectal cancer and which are present in altered states but not necessarily driving disease progression.
Read More...Impact of gadodiamide (Omniscan) on a beef liver catalase ex vivo model
Here, seeking to better understand the effects of gadolinium-based contrast agents, dyes typically used for MRI scans, the authors evaluated the activity of catalase found in beef liver both with and without gadodiamide when exposed to hydrogen peroxide. They found that gadioamide did not significantly inhibit catalase's activity, attributing this lack of effects to the chelating agent found in gadodiamide.
Read More...Post-Traumatic Stress Disorder (PTSD) biomarker identification using a deep learning model
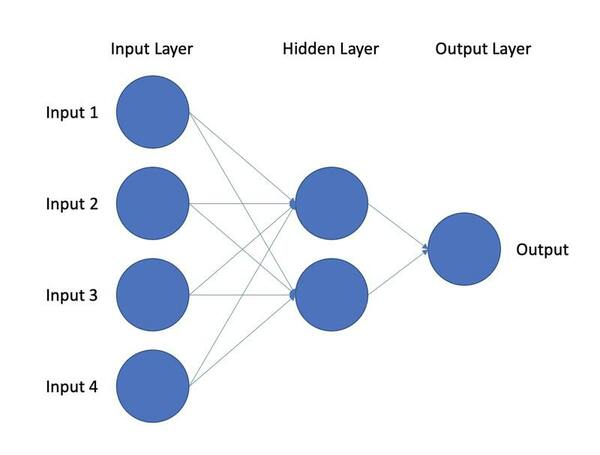
In this study, a deep learning model is used to classify post-traumatic stress disorder patients through novel markers to assist in finding candidate biomarkers for the disorder.
Read More...Modular mimics of neuroactive alkaloids - design, synthesis, and cholinesterase inhibitory activity of rivastigmine analogs
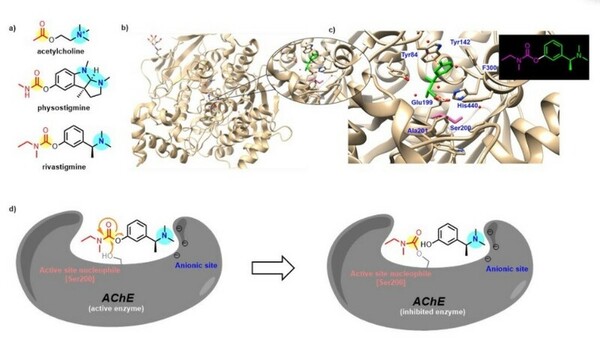
Naturally occurring neuroactive alkaloids are often studied for their potential to treat Neurological diseases. This team of students study Rivastigmine, a potent cholinesterase inhibitor that is a synthetic analog of physostigmine, which comes from the Calabar bean plant Physostigma venenosum. By comparing the effects of optimized synthetic analogs to the naturally occurring alkaloid, they determine the most favorable analog for inhibition of acetylcholinesterase (AChE), the enzyme that breaks down the neurotransmitter acetylcholine (ACh) to terminate neuronal transmission and signaling between synapses.
Read More...