A colorimetric investigation of copper(II) solutions
(1) Milton Academy, Milton, Massachusetts
https://doi.org/10.59720/22-284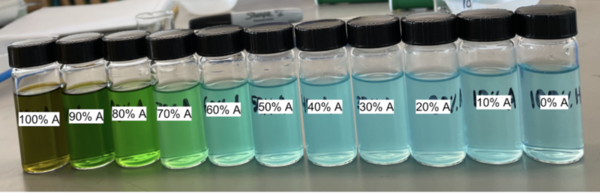
Complex ions containing transition metals, elements with incompletely filled d-orbitals, are usually colored, while similar ions from non-transition metals are not. Copper (Cu) is a transition metal with a flexible coordination sphere, meaning it can adopt many coordination geometries, and therefore have various colored complexes. Addition of Chloride (Cl-) to a CuCl2 solution causes color change away from blue toward a yellowish green. We hypothesized that adding acetone to an CuCl2-water solution would cause a similar shift in solution color. We made aqueous CuCl2 solutions with increasing acetone concentrations ranging from 0 to 100 percent and compared the resulting colors to aqueous CuCl2 solutions with increasing added amounts of NaCl to find the closest visual fit in color from the acetone solutions for each added NaCl equivalent. Our experiment showed that increasing the percent acetone in aqueous CuCl2 solutions gave rise to a color change parallel to that of adding Cl-, shifting solution color away from blue and toward yellow. We propose an explanation of this relationship by considering Cu2+ reduction in high acetone concentrations and discussing ligand field strength and the possible effects of tetrahedral versus octahedral geometries on splitting: weaker splitting may similarly affect color through equilibria leaning towards green/yellow products. This investigation could support our understanding of copper coordination and copper complex color behavior with future implications for other copper complex experiments or, in a broader perspective, detection and evaluation of Cu2+ levels in environmental and biological systems.
This article has been tagged with: