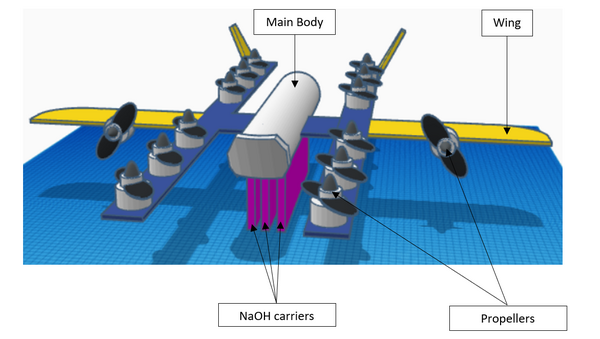
In this study, the authors address the current climate concern of high CO2 levels by testing solid forms of hydroxide for CO2 reduction and designing a drone to fly it in ambient air!
Read More...Use of drone with sodium hydroxide carriers to absorb carbon dioxide from ambient air

In this study, the authors address the current climate concern of high CO2 levels by testing solid forms of hydroxide for CO2 reduction and designing a drone to fly it in ambient air!
Read More...A comparison of starches and plasticizers for biopolymer synthesis and degradation
Improving measurement of reducing sugar content in carbonated beverages using Fehling’s reagent

The sugar-rich modern diet underlies a suite of metabolic disorders, most common of which is diabetes. Accurately reporting the sugar content of pre-packaged food and drink items can help consumers track their sugar intake better, facilitating more cognisant and, eventually, moderate consumption of high-sugar items. In this article, the authors examine the effect of several variables on the accuracy of Fehling's reaction, a colorimetric reaction used to estimate sugar content.
Read More...Managing CO2 levels through precipitation-based capture from seawater and electrochemical conversion
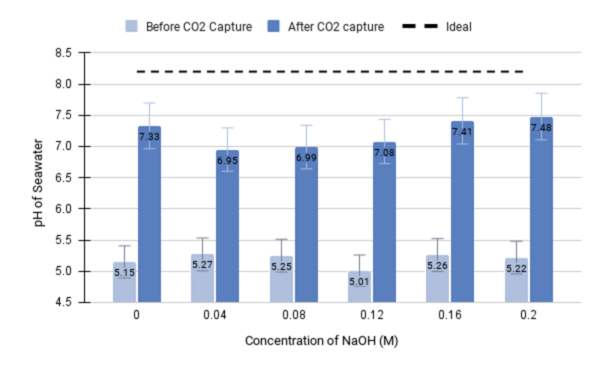
The authors set out to develop an electrochemical device that would have efficient and sustained carbon dioxide capture.
Read More...Lactic acid bacteria protect the growth of Solanum lycopersicum from Sodium dodecyl sulfate
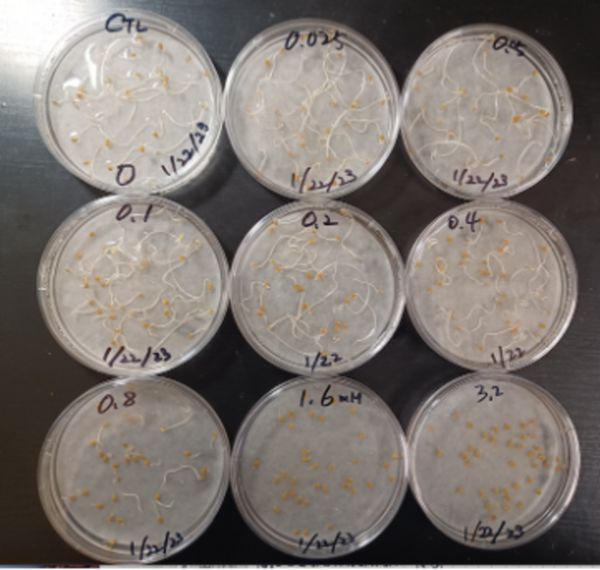
Sodium dodecyl sulfate (SDS), a detergent component, can harm plant growth when it contaminates soil and waterways. Authors explored the potential of lactic acid bacteria (LAB) to mitigate SDS-induced stress on plants.
Read More...Synthesis of sodium alginate composite bioplastic films

The authors looked at the development of biodegradable bioplastic and its features compared to PET packaging films. They were able to develop a biodegradable plastic with sodium alginate that dissolved in water and degrade in microbial conditions while also being transparent and flexible similar to current plastic films.
Read More...The Effect of Concentration on the Pressure of a Sodium Chloride Solution Inside Dialysis Tubing
In this study, the authors investigate the effects of sodium levels on blood pressure, one of the most common medical problems worldwide. They used a simulated blood vessel constructed from dialysis tubing to carefully analyze pressure changes resulting from various levels of sodium in the external solution. They found that when the sodium concentration in the simulated blood vessel was higher than the external fluid, internal pressure increased, while the reverse was true when the sodium concentration was lower than in the surrounding environment. These results highlight the potential for sodium concentration to have a significant effect on blood pressure in humans by affecting the rate of osmosis across the boundaries of actual blood vessels.
Read More...Energy beverages and sugar: How sweetener type dictates specific gravity
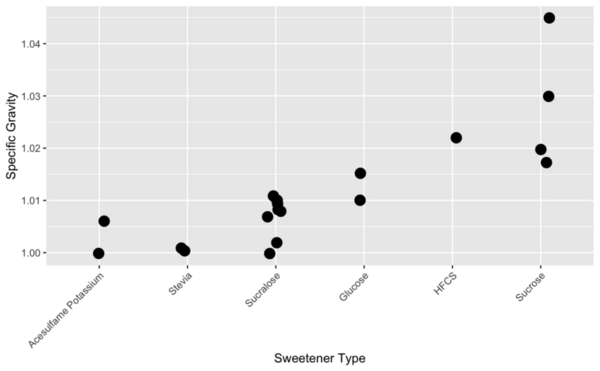
The authors looked at different factors that influence specific gravity in beverages, including sweetener used, caffeine, carbonation, and sodium.
Read More...Osmotic characteristics of water retention structures of Bursera microphylla in relation to soil salinity

This study hypothesized that sodium chloride was taken up through plant root structures to facilitate water transportation, and that sodium chloride accumulation was directly proportional to the soil salinity. Results showed that most cells within the “bulb” structures were isotonic at a concentration approximately twice as high as that of root tissue and ambient soil salinity, therefore supporting the presented hypothesis.
Read More...Buttermilk and baking soda increase pancake fluffiness by liberating carbon dioxide

Here, seeking a better understanding of what determines the fluffiness of a pancake, the authors began by considering a chemical reaction that results in the production of carbon dioxide gas from recipe ingredients, specifically sodium bicarbonate or baking soda. The substitution of homemade buttermilk for milk and adding more baking soda was found to result in significantly fluffier pancakes.
Read More...