Managing CO2 levels through precipitation-based capture from seawater and electrochemical conversion
(1) Amador Valley High School, Pleasanton, California, (2) Department of Chemical and Biomedical Engineering, University of Iowa, Iowa City, Iowa
https://doi.org/10.59720/22-060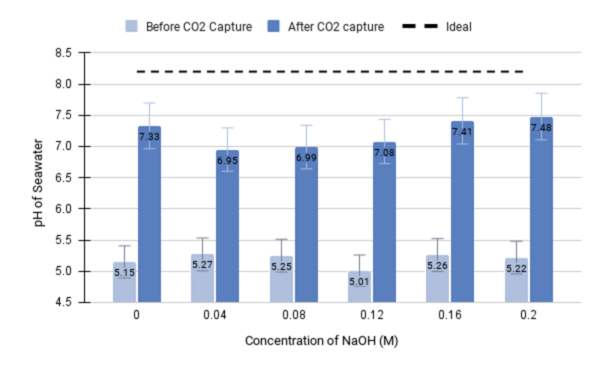
With the rise in global greenhouse gas emissions, many studies have sought methods to reduce the accumulation of carbon dioxide (CO2) in the atmosphere. Existing methodologies are primarily based on amine solvents such as monoethanolamine (MEA) and 2-amino-2-methylpropanol (AMP) due to their high carbon absorption capacity and good recyclability. As such, they are used for the capture of CO2 from sequestration sites and its subsequent electrochemical transformation. However, because amines have large enthalpies of reaction, this leads to expensive and energy-intensive processes that limit their capacity for large-scale application. Thus, this study seeks to develop an electrochemical device that can sustain efficient CO2 capture and conversion through precipitation. The dissolved inorganic divalent cation calcium (Ca,2+) reacts with carbon to form carbonate (CO32-) compounds that flow through a thin membrane, facilitating the filtration of the precipitate. We hypothesized that higher alkalinity will produce a greater quantity of CO2 capture, as measured by the change in seawater pH and the mass of precipitate formed. The CO32- compound is, in turn, converted to the industrial fuel formic acid (HCOOH). Through our experimentation, we found a statistically significant difference between the measured pH of seawater before and after CO2 capture (p < 0.001), thus suggesting this setup as a proof of concept for the effective reduction of ocean acidification. Additionally, we observed an upwards trend as higher concentrations of sodium hydroxide (NaOH) were generally associated with greater changes in pH.
This article has been tagged with: