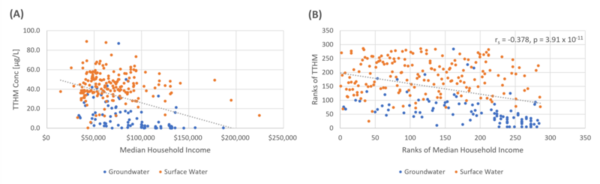
Trihalomethanes, probable human carcinogens, are commonly found disinfection by-products (DBPs) in public water systems (PWS). The authors investigated the correlation between trihalomethane concentrations and socioeconomic factors in New York State, finding a negative correlation between median household income and trihalomethane concentrations. The inverse association between trihalomethanes and household income may indicate socioeconomic disparity regarding drinking water quality and the need for improved efforts to assist small- and medium-sized community water systems to lower DBP levels in New York State.
Read More...