.jpg)
In this study, the ability of arbuscular mycorrhizal fungi to limit the growth of an agricultural weed Cirsium arvense is tested. This has important implications for developing natural herbicides.
Read More...Application of arbuscular mycorrhizal fungi to inhibit nitrogen uptake of weeds within crop fields
.jpg)
In this study, the ability of arbuscular mycorrhizal fungi to limit the growth of an agricultural weed Cirsium arvense is tested. This has important implications for developing natural herbicides.
Read More...Investigating momentum transfer with gall-forming wasps
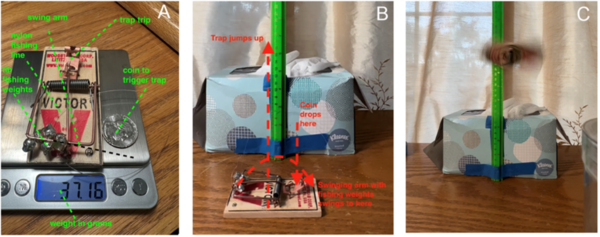
The authors use the unique movements of the jumping gall wasp to study momentum transfer with potential applications in robotics and extraterrestrial research.
Read More...Longer Exposure to 2% India Ink Increases Average Number of Vacuoles in Tetrahymena pyriformis
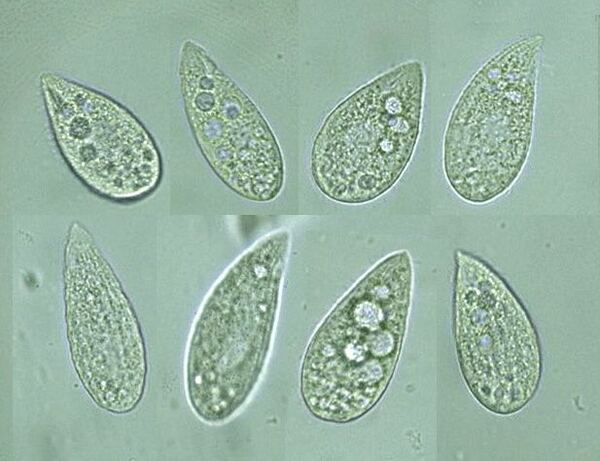
Phagocytes feed by forming food vacuoles. In this article the authors investigate the extent that exposure of non-nutritional food, such as India Ink, to Tetrahymena pyriformis affects the number of vacuole formation. These studies provide insight to how organisms budget their energy and metabolic processes during an energy shortage.
Read More...Survival of Escherichia coli K-12 in various types of drinking water

For public health, drinking water should be free of bacterial contamination. The objective of this research is to identify the fate of bacteria if drinking water becomes contaminated and inform consumers on which water type enables the least bacteria to survive. We hypothesized that bottled mineral water would provide the most sufficient conditions for E. coli to survive. We found that if water becomes contaminated, the conditions offered by the three water types at room temperature allow E. coli to survive up to three days. At 72 hours, the bottled spring water had the highest average colony forming units (CFUs), with tap and mineral water CFU values statistically lower than spring water but not significantly different from each other. The findings of this research highlight the need of implementing accessible quality drinking water for the underserved population and for the regulation of water sources.
Read More...Evaluating cinnamaldehyde as an antibacterial agent in a produce wash for leafy greens

Recognizing a growing demand for organic produce, the authors sought to investigate plant-based antibiotic solutions to meet growing consumer demand for safe produce and also meet microbial standards of the USDA. The authors investigated the use of cinnamaldehyde as an antibacterial again E. coli, finding that lettuce treated with cinnamaldehyde displayed significantly lower colony-forming units of E. coli when compared to lettuce treated with chlorine bleach.
Read More...Investigating the Role of Biotic Factors in Host Responses to Rhizobia in the System Medicago truncatula
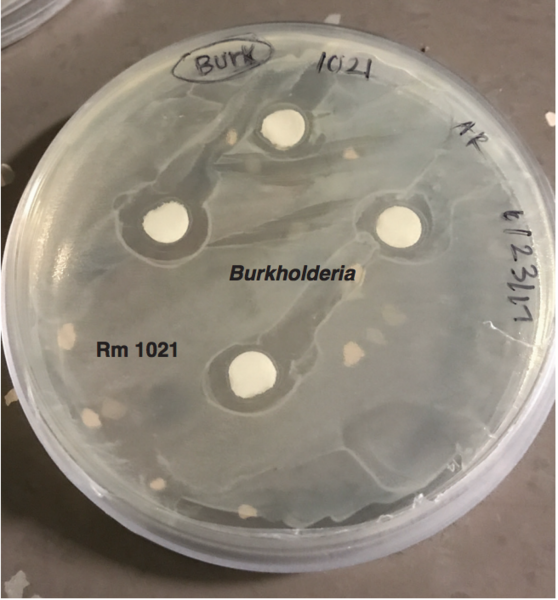
Nitrogen-fixing bacteria, such as the legume mutualist rhizobia, convert atmospheric nitrogen into a form that is usable by living organisms. Leguminous plants, like the model species Medicago truncatula, directly benefit from this process by forming a symbiotic relationship with rhizobia. Here, Rathod and Rowe investigate how M. truncatula responds to non-rhizobial bacterial partners.
Read More...The effects of UV-C and ionizing radiation on the functions of Escherichia Coli

In this study, the authors send E. coli cultures to space via the Cubes in SpaceTM program to determine if ultraviolet C and ionizing radiation negatively affect bacterial growth.
Read More...Increased carmine red exposure periods yields a higher number of vacuoles formed in Tetrahymena pyriformis
.jpeg)
T. pyriformis can use phagocytosis to create vacuoles of carmine red, a dye which is made using crushed insects and is full of nutrients. Establishing a relationship between vacuole formation and duration of exposure to food can demonstrate how phagocytosis occurs in T. pyriformis. We hypothesized that if T. pyriformis was incubated in a carmine red solution, then more vacuoles would form over time in each cell.
Read More...Disputing the green valley theory of galaxy evolution
Converting SiO2 wafers to hydrophobic using chlorotrimethylsilane
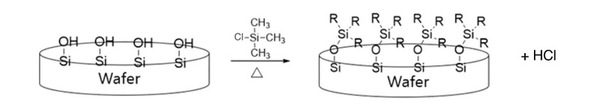
Semiconductors are the center of the fourth industrial revolution as they are key components for all electronics. Exposed wafers made of silicon (Si), which can easily oxidize, convert to silicon dioxide (SiO2). The surface of SiO2 wafers consists of many Si-OH bonds, allowing them to easily bond with water, resulting in a “wet” or hydrophilic condition. We sought to determine a way to modify the surface of SiO2 wafers to become hydrophobic to ensure safe wet cleaning.
Read More...