Converting SiO2 wafers to hydrophobic using chlorotrimethylsilane
(1) Westview High School, (2) Irvine High School, (3) Westside High School, (4) Portola High School, (5) Gretchen Whitney High School, (6) Cerritos High School, (7) James M Bennett, (8) Dutch Fork High School, (9) Lakeridge High School, (10) Intel Corporation
https://doi.org/10.59720/23-100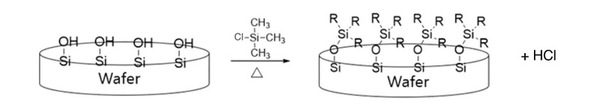
Semiconductors are the center of the fourth industrial revolution as they are key components for all electronics. To manufacture chips, semiconductor companies purchase bare wafers and perform many different processes to ensure the finest quality of each chip. Exposed wafers made of silicon (Si), which can easily oxidize, convert to silicon dioxide (SiO2). The surface of SiO2 wafers consists of many Si-OH bonds, allowing them to easily bond with water, resulting in a “wet” or hydrophilic condition. The hydrophilic condition of a wafer can be problematic as the fin can collapse during water rinsing. We sought to determine a way to modify the surface of SiO2 wafers to become hydrophobic to ensure safe wet cleaning. We hypothesized that treating the surface of the wafer with hydrophobic chemicals like chlorotrimethylsilane (CTS) could address this problem by converting the surface of the wafer into Si-OR (R group being hydrocarbons). This conversion would prevent hydrogen bonds from forming with water, which would then convert the wafer from hydrophilic to hydrophobic. After executing the experiment, the hydrophobicity was analyzed by measuring the contact angle of the wafer, where increases in contact angle portrayed a more hydrophobic surface. In our results, there was an increase in contact angle, which concluded that coating the SiO2 wafer with CTS repels water from the surface, therefore allowing for a safe cleansing process. The prevalence of semiconductors today makes it especially important for maximum efficiency in the industry; a rinsing process minimizing risks would curtail costs and time.
This article has been tagged with: