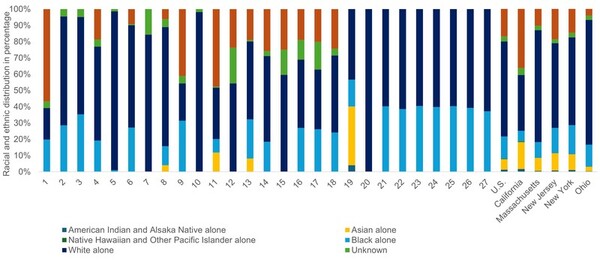
The authors analyzed racial and ethnic representation in studies on PFAS and neurological health outcomes.
Read More...Population demographic patterns in PFAS-neurological health research

The authors analyzed racial and ethnic representation in studies on PFAS and neurological health outcomes.
Read More...Predicting clogs in water pipelines using sound sensors and machine learning linear regression
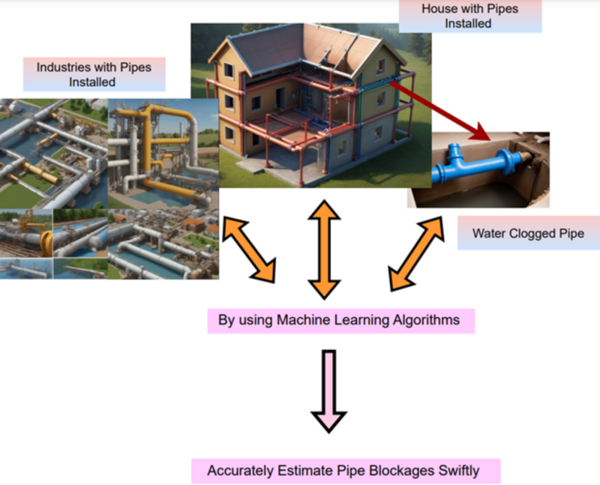
The authors looked the ability of sound sensors to predict clogged pipes when the sound intensity data is run through a machine learning algorithm.
Read More...Effect of hypervitaminosis A in regenerating planaria: A potential model for teratogenicity testing
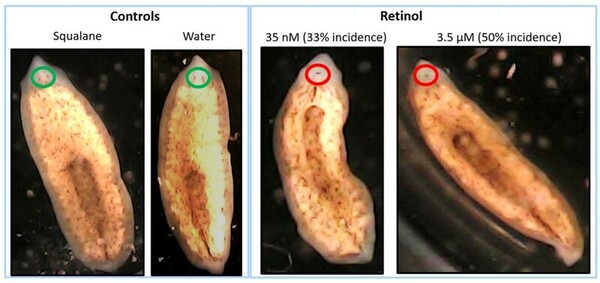
This unique research study evaluated the potential use of the flatworm, brown planaria (Dugesia tigrine), as an alternative model for teratogenicity testing. In this study, we exposed amputated planaria to varying concentrations of a known teratogen, vitamin A (retinol), for approximately 2 weeks, and evaluated multiple parameters including the formation of blastema and eyes. The results from this study demonstrated that high concentrations of retinol caused defects in head and eye formation in regenerating planaria, with similarities to vitamin A related teratogenicity findings in mammals. Based on these results, regenerating brown planaria are a promising alternative model for teratogenicity testing, which can potentially be paradigm shifting as it can reduce cost, time, and pregnant animal use in research.
Read More...Innovative Treatment for Reducing Senescence and Revitalizing Aging Cells through Gene Silencing
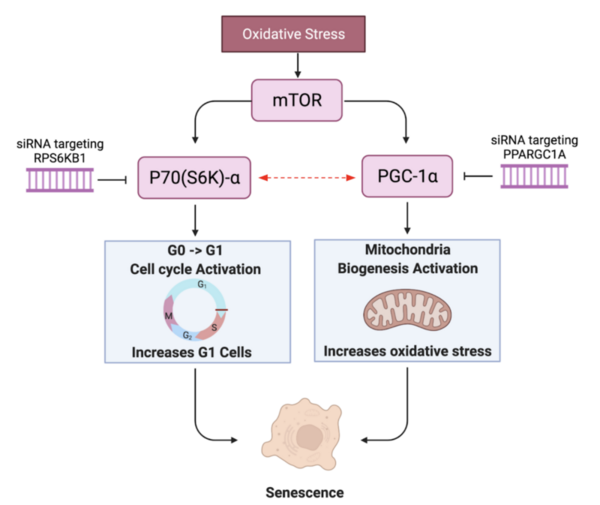
Cellular senescence plays a key role in aging cells and is attributed to a number of disease and pathology. These authors find that genetic editing of both RPS6KB1 and PPARGC1A revitalizes a human skin fibroblast cell line.
Read More...Computational evidence for differential endocrine disruption by DEHP and PET via estrogen receptor beta binding
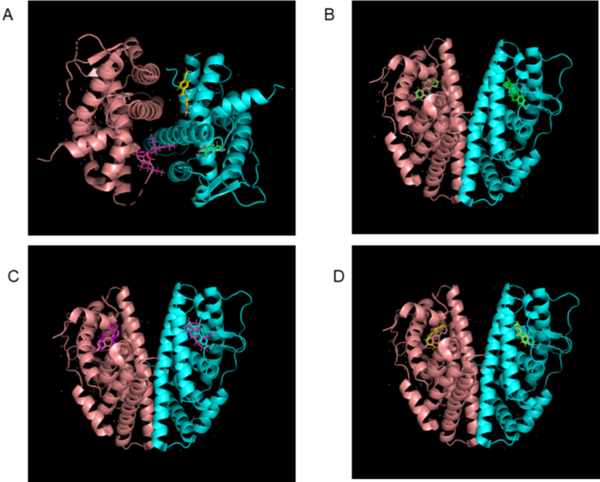
The authors demonstrate computationally that two types of nanoplastics can bind to estrogen receptor beta and potentially disrupt endocrine systems.
Read More...Computational analysis and drug repositioning: Targeting the TDP-43 RRM using FDA-approved drugs
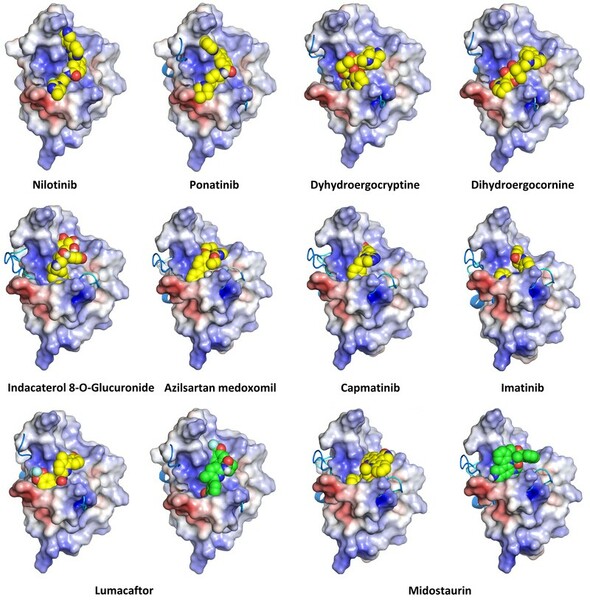
Molecules which bind to proteins that aggregate abnormally in neurodegenerative diseases could be promising drugs for these diseases. In this study, Zhang, Wu, Zhang, and Dang simulate the binding behavior of various molecules to screen for candidates which could be promising candidates for drug development.
Read More...Household spices and minerals as alternative disinfectants for mobile phones

In this study, the authors investigate the disinfectant potential of many household spices and minerals. More specifically, they test whether these compounds can be used to disinfect mobile phones after daily use with the hope of identifying environmentally-friendly cleaning options.
Read More...The effect of nicotine and lead on neuron morphology, function, and ɑ-Synuclein levels in a C. elegans model
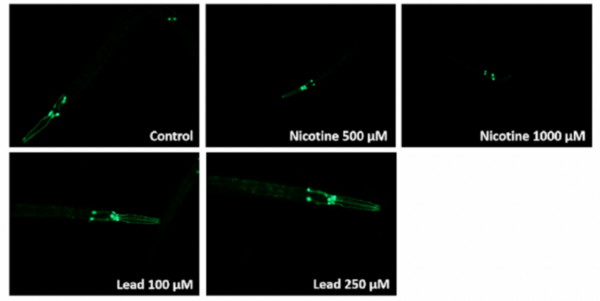
E-cigarettes are often considered a healthier alternative to traditional cigarettes. This team of high school authors investigated the impact of common e-cigarette compounds on C. elegans, and found a number of harmful effects ultimately resulting in injury and neuronal damage.
Read More...Comparing the effects of electronic cigarette smoke and conventional cigarette smoke on lung cancer viability

Here, recognizing the significant growth of electronic cigarettes in recent years, the authors sought to test a hypothesis that three main components of the liquid solutions used in e-cigarettes might affect lung cancer cell viability. In a study performed by exposing A549 cells, human lung cancer cells, to different types of smoke extracts, the authors found that increasing levels of nicotine resulted in improve lung cancer cell viability up until the toxicity of nicotine resulted in cell death. They conclude that these results suggest that contrary to conventional thought e-cigarettes may be more dangerous than tobacco cigarettes in certain contexts.
Read More...The Effects of Barley Straw (Hordeum vulgare) Extract and Barley Straw Pellets on Algal Growth and Water Quality

Algal overgrowth often threatens to clog irrigation pipes and drinking water lines when left unchecked, as well as releasing possible toxins that threaten plant and human health. It is thus important to find natural, non-harmful agents that can decrease algal growth without threatening the health of plants and humans. In this paper, the authors test the efficacy of barely extract in either liquid or pellet form in decreasing algal growth. While their results were inconclusive, the experimental set-up allows them to investigate a wider range of agents as anti-algal treatments that could potentially be adopted on a wider scale.
Read More...