Effect of hypervitaminosis A in regenerating planaria: A potential model for teratogenicity testing
(1) Hopkinton High School, Hopkinton, MA, (2) Alexion Pharmaceuticals Inc., Boston, MA
https://doi.org/10.59720/22-066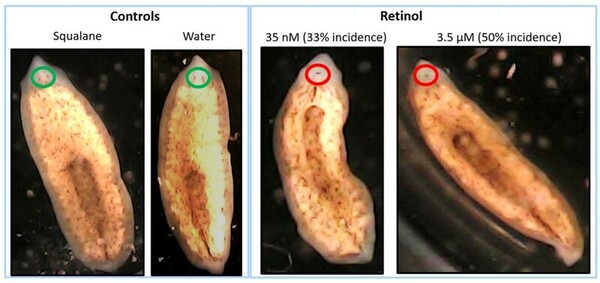
According to the Centers for Disease Control and Prevention, a child with birth defects is born approximately every 4.5 minutes. The currently accepted method to determine the potential of new medicines and chemicals to cause birth defects (teratogenicity) involves testing in pregnant animals. Millions of mammalian animals are sacrificed annually for research, and scientists are searching for alternative models to reduce mammal use in research. This unique research study evaluated the potential use of the flatworm, brown planaria (Dugesia tigrine), as an alternative model for teratogenicity testing. There are similarities between mammalian embryonic development and regeneration of planaria during the early stages of development of the nervous system, including the eyes. In this study, we exposed amputated planaria to varying concentrations of a known teratogen, vitamin A (retinol), for approximately 2 weeks, and evaluated multiple parameters including the formation of blastema and eyes. Retinol delayed the formation of blastema and eyes in regenerating planaria in a concentration-dependent manner. At high concentrations of retinol, regenerating planaria also developed cyclopia, a well-known birth defect in humans, which has been linked to high levels of vitamin A in mammalian studies. The results from this study demonstrated that high concentrations of retinol caused defects in head and eye formation in regenerating planaria, with similarities to vitamin A related teratogenicity findings in mammals. Based on these results, regenerating brown planaria are a promising alternative model for teratogenicity testing, which can potentially be paradigm shifting as it can reduce cost, time, and pregnant animal use in research.
This article has been tagged with: