Computational evidence for differential endocrine disruption by DEHP and PET via estrogen receptor beta binding
(1) George W. Hewlett High School, (2) Virginia Commonwealth University, School of Medicine
https://doi.org/10.59720/25-196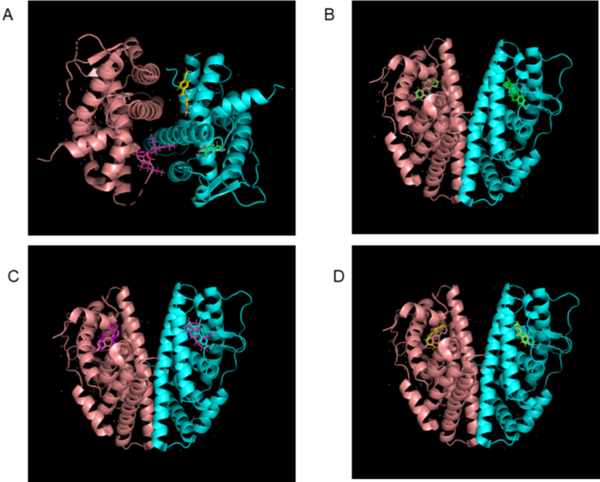
Nanoplastics, particularly those derived from polyethylene terephthalate (PET) and polyvinyl chloride (PVC), represent a growing environmental concern with potential impacts on human health through endocrine disruption. This study investigated the binding interactions between two nanoplastic models—di-(2-ethylhexyl) phthalate (DEHP, representing PVC) and a PET monomer—and human estrogen receptor beta (ERβ) using computational docking experiments. We hypothesized that both compounds would demonstrate strong binding affinities to ERβ, potentially disrupting normal estrogen signaling. Using DockThor for docking simulations, we found that DEHP exhibited binding affinities comparable to the native ligand estradiol (chain A: -10.02 ± 0.23 kcal/mol; chain B: -10.07 ± 0.22 kcal/mol), while PET showed significantly weaker binding (chain A: -8.25 ± 0.05 kcal/mol; chain B: -8.15 ± 0.05 kcal/mol). Toxicity predictions from ProTox and Virtual models for property Evaluation of chemicals within a Global Architecture (VEGA) platform corroborated these findings, with DEHP showing higher likelihoods of endocrine disruption and PET demonstrating minimal impact. Analysis of root mean square deviation (RMSD) values revealed that both compounds induced conformational changes in ERβ similar to estradiol, with chain-specific differences observed. These findings suggest that DEHP poses a greater risk for endocrine disruption, while PET's weaker and more variable binding indicates lower potential for direct receptor interference. This study provides computational evidence for differential endocrine disruption potential among nanoplastics and highlights the need for experimental validation through in vitro and in vivo studies.
This article has been tagged with: