
This study compares different methods for cooking vegetables to determine which retain iron and ascorbic acid, or vitamin C, levels the most.
Read More...Effect of different cooking methods on the levels of iron and ascorbic acid in green vegetables

This study compares different methods for cooking vegetables to determine which retain iron and ascorbic acid, or vitamin C, levels the most.
Read More...Detection method of black goji berry anthocyanin content based on colorimetry
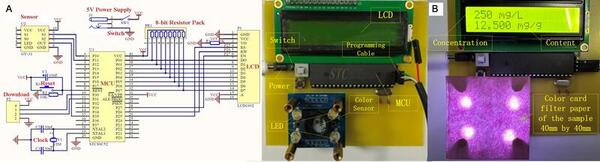
Black goji berries have attracted interest for their high levels of anthocyanin pigment, which believed to have health-boosting effects. Yu and Zhu research a method for measuring goji berry quality by detecting anthocyanin content under different conditions.
Read More...The effect of an anthocyanin on the gut permeability of a Type 2 Diabetic Drosophila melanogaster

Anti-diabetic drugs like Metformin are known to increase gut permeability, and this has a negative impact on patient health. These authors hypothesized that this can be mitigated using purple sweet potato extract, which is high anthocyanin content, that feeds bacteria metabolism to decrease gut permeability.
Read More...Efficient synthesis of superabsorbent beads using photopolymerization with a low-cost method
Superabsorbent beads are remarkable, used throughout our daily lives for various practical applications. These beads, as suggested by their name, possess a unique ability to absorb and retain large quantities of liquids. This characteristic of absorbency makes them essential throughout the medical field, agriculture, and other critical industries as well as in everyday products. To create these beads, the process of photopolymerization is fast growing in favor with distinct advantages of cost efficiency, speed, energy efficiency, and mindfulness towards the environment. In this article, researchers explore the pairing of cheap monomers with accessible equipment for creation of superabsorbent beads via the photopolymerization process. This research substantially demonstrates the successful application of photopolymerization in producing highly absorbent beads in a low-cost context, thereby expanding the accessibility of this process for creating superabsorbent beads in both research and practical applications.
Read More...Optimizing surface contact area and electrolyte type to develop a more effective rechargeable battery
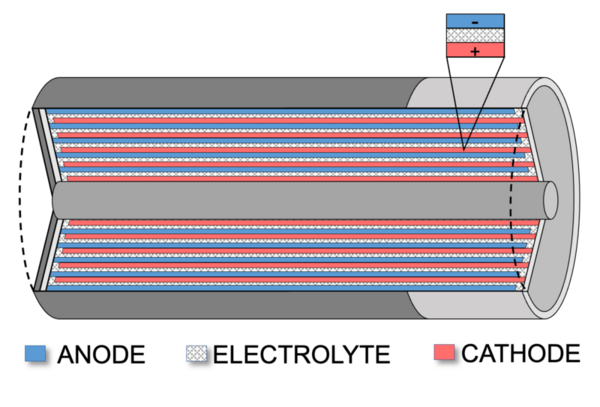
Rechargeable batteries are playing an increasingly prominent role in our lives due to the ongoing transition from fossil energy sources to green energy. The purpose of this study was to investigate variables that impact the effectiveness of rechargeable batteries. Alkaline (non-rechargeable) and rechargeable batteries share common features that are critical for the operation of a battery. The positive and negative electrodes, also known as the cathode and anode, are where the energy of the battery is stored. The electrolyte is what facilitates the transfer of cations and anions in a battery to generate electricity. Due to the importance of these components, we felt that a systematic investigation examining the surface area of the cathode and anode as well the impact of electrolytes with different properties on battery performance was justified. Utilizing a copper cathode and aluminum anode coupled with a water in salt electrolyte, a model rechargeable battery system was developed to test two hypotheses: a) increasing the contact area between the electrodes and electrolyte would improve battery capacity, and b) more soluble salt-based electrolytes would improve battery capacity. After soaking in an electrolyte solution, the battery was charged and the capacity, starting voltage, and ending voltage of each battery were measured. The results of this study supported our hypothesis that larger anode/cathodes surface areas and more ionic electrolytes such as sodium chloride, potassium chloride and potassium sulfate resulted in superior battery capacity. Incorporating these findings can help maximize the efficiency of commercial rechargeable batteries.
Read More...Correlation between trihalomethane concentrations and various cancers in Massachusetts counties
.png)
The authors assess incidence and mortality rates of two cancer types in relation to trihalomethane pollutant concentrations in drinking water.
Read More...Water tubing injury patterns among different demographics: A NEISS study

The authors looked at how injuries sustained during water tubing, that require treatment at an emergency department, differ between males and females.
Read More...Analysis of electrodialysis as a method of producing potable water
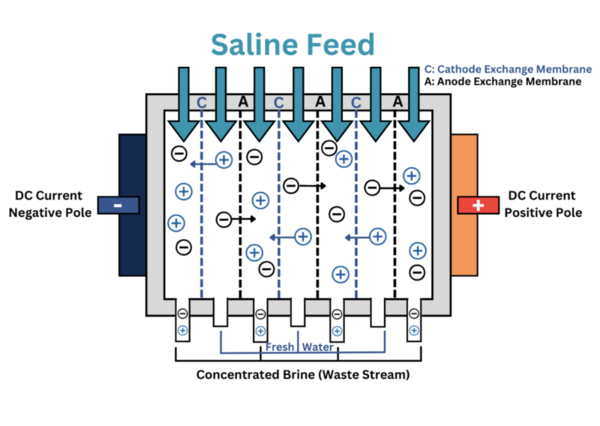
Here, seeking a way to convert the vast quantity of seawater to drinking water, the authors investigated the purification of seawater to drinking water through electrodialysis. Using total dissolved solids (TDS) as their measure, they found that electrodialysis was able to produce deionized water with TDS values under the acceptable range for consumable water.
Read More...Heavy metal and bacterial water filtration using Moringa oleifera and coconut shell-activated carbon
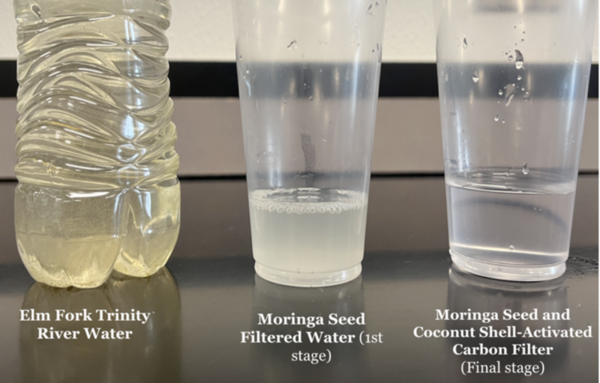
One-third of the world's people do not have access to clean drinking water. Nadella and Nadella tackle this issue by testing a low-cost filtration system for removing heavy metal and bacteria from water.
Read More...A comparison of the water quality between Chinatown and Bayside: two demographically different regions
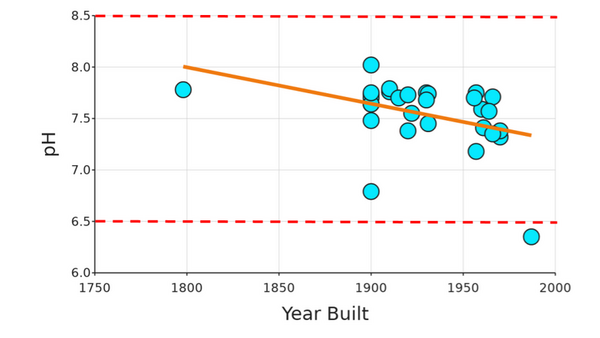
The authors looked at differences in water quality between Chinatown and Bayside. They wanted to look at the racial and economic demographics of each region and how that correlated to access to clean drinking water. Ultimately they did not find any significant differences in water quality, but identified important future directions for this work.
Read More...