Efficient synthesis of superabsorbent beads using photopolymerization with a low-cost method
(1) Franklin Regional Senior High School, Murrysville, PA, (2) Department of Chemistry, Saint Vincent College, Latrobe, PA
https://doi.org/10.59720/22-172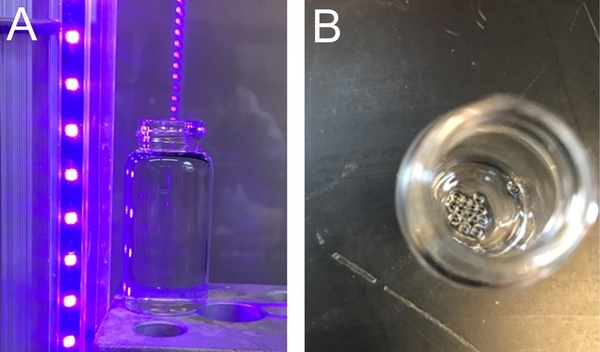
Superabsorbent polymers are widely used in hygiene, agriculture, and contamination removal products. The preparation of superabsorbent beads has been reported in the literature from thermal and photopolymerizations of hydrophilic monomers. The rate of photopolymerization is normally faster than thermal polymerization. Our study aimed to test whether the fast photopolymerization technique would work with cheap, easily accessible monomers, and simple equipment that is affordable to high school students within a typical chemistry lab setting. We also evaluated the water absorbance of polymers prepared from hydrophilic monomers. We chose two hydrophilic monomers, N,N’-dimethylacrylamide (DMAA) and N-hydroxyethyl acrylamide (HEAA), and employed LED UV/Vis light as the light source to break photoinitiator molecules, with silicone oil as the polymerization medium. We hypothesized that superabsorbent beads could be prepared from DMAA and HEAA using the fast photopolymerization technique at room temperature using silicone oil. Our experiments demonstrated the synthesis of superabsorbent beads from a mixture of DMAA, HEAA, and photoinitiator in 60 seconds at ambient conditions. We also discovered that water absorbance increased with the increase of DMAA when its concentration was equal to or less than 25% by weight in the monomer mixture. Beads were completely soluble in water when the concentration of DMAA was equal to or over 50% by weight. Beads with a 25:75 DMAA:HEAA weight ratio demonstrated the highest efficiency among the six compositions tested. Our research also paves the way for high school students to investigate advanced polymerizations in a very simple way.
This article has been tagged with: