
In this article the authors looked at how temperature was impacted when alumnium was added in various forms to aqueous copper(II) solutions. Their study investigates the impact of surface area on chemical reactions.
Read More...Impact of aluminum surface area on the rate of reaction with aqueous copper (II) chloride solutions

In this article the authors looked at how temperature was impacted when alumnium was added in various forms to aqueous copper(II) solutions. Their study investigates the impact of surface area on chemical reactions.
Read More...A colorimetric investigation of copper(II) solutions
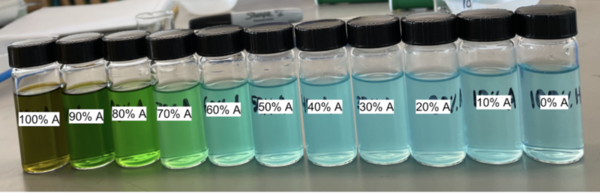
In this study, the authors investigate the effects of acetone on the color of copper chloride (CuCl2) solution, which has important implications for detecting copper in the environment.
Read More...Optimizing surface contact area and electrolyte type to develop a more effective rechargeable battery
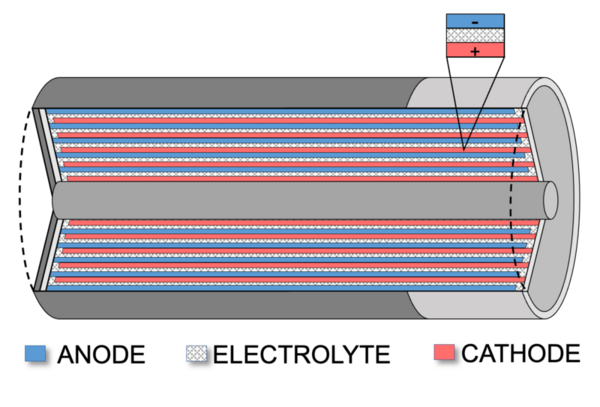
Rechargeable batteries are playing an increasingly prominent role in our lives due to the ongoing transition from fossil energy sources to green energy. The purpose of this study was to investigate variables that impact the effectiveness of rechargeable batteries. Alkaline (non-rechargeable) and rechargeable batteries share common features that are critical for the operation of a battery. The positive and negative electrodes, also known as the cathode and anode, are where the energy of the battery is stored. The electrolyte is what facilitates the transfer of cations and anions in a battery to generate electricity. Due to the importance of these components, we felt that a systematic investigation examining the surface area of the cathode and anode as well the impact of electrolytes with different properties on battery performance was justified. Utilizing a copper cathode and aluminum anode coupled with a water in salt electrolyte, a model rechargeable battery system was developed to test two hypotheses: a) increasing the contact area between the electrodes and electrolyte would improve battery capacity, and b) more soluble salt-based electrolytes would improve battery capacity. After soaking in an electrolyte solution, the battery was charged and the capacity, starting voltage, and ending voltage of each battery were measured. The results of this study supported our hypothesis that larger anode/cathodes surface areas and more ionic electrolytes such as sodium chloride, potassium chloride and potassium sulfate resulted in superior battery capacity. Incorporating these findings can help maximize the efficiency of commercial rechargeable batteries.
Read More...Tap water quality analysis in Ulaanbaatar City

There have been several issues concerning the water quality in Ulaanbaatar, Mongolia in the past few years. This study, we collected 28 samples from 6 districts of Ulaanbaatar to check if the water supply quality met the standards of the World Health Organization, the Environmental Protection Agency, and a Mongolian National Standard. Only three samples fully met all the requirements of the global standards. Samples in Zaisan showed higher hardness (>120 ppm) and alkalinity levels (20–200 ppm) over the other districts in the city. Overall, the results show that it is important to ensure a safe and accessible water supply in Ulaanbaatar to prevent future water quality issues.
Read More...Copper nanoparticle synthesis using Picea glauca ‘Conica’
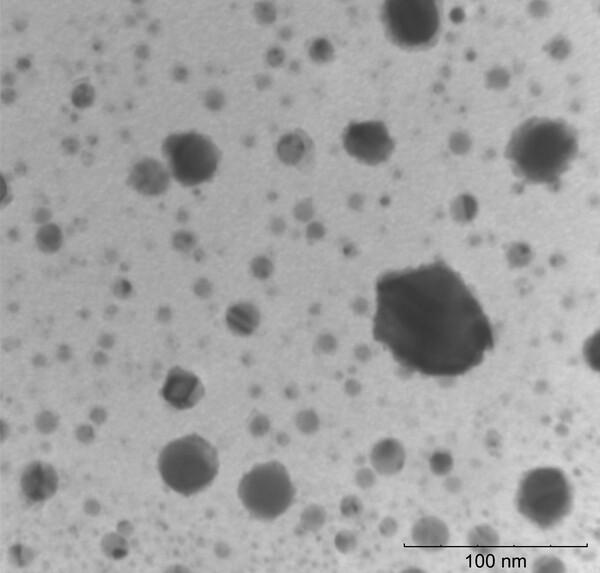
The authors propose a method to recycle Christmas tree needles into a non-toxic reducing agent for synthesizing copper nanoparticles.
Read More...Effects of copper sulfate exposure on the nervous system of the Hirudo verbana leech
In this study, the authors test whether excess copper exposure has neurobehavioral effects on Hirudo verbana leeches.
Read More...The effect of molecular weights of chitosan on the synthesis and antifungal effect of copper chitosan
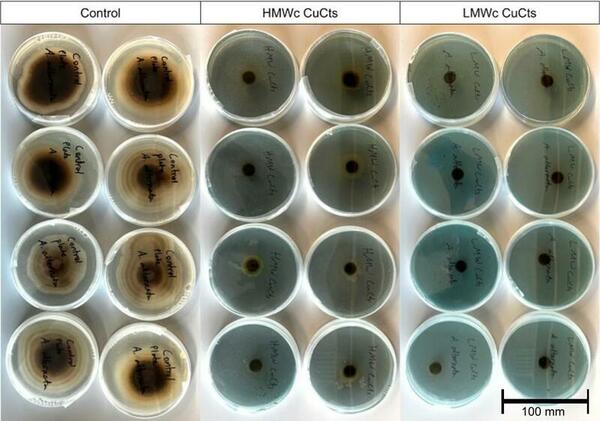
Pathogenic fungi such as Alternaria alternata (A. alternata) can decimate crop yields and severely limit food supplies when left untreated. Copper chitosan (CuCts) is a promising alternative fungicide for developing agricultural areas due to being inexpensive and nontoxic. We hypothesized that LMWc CuCts would exhibit greater fungal inhibition due to the beneficial properties of LMWc.
Read More...Antibacterial Effects of Copper Surfaces
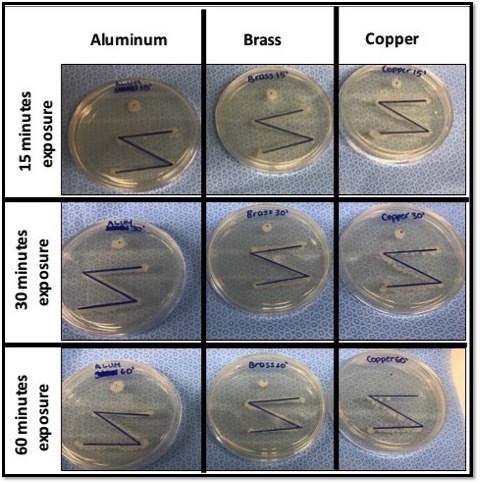
This study examined the ability of copper and copper alloy surfaces to inhibit bacterial growth, which may be help prevent healthcare-associated infections. The authors exposed two non-pathogenic strains of bacteria to different metal plates for varying degrees of time and measured bacterial growth.
Read More...Differences in the effect of copper sulfate on the mortality rate of Ostracod and Daphnia
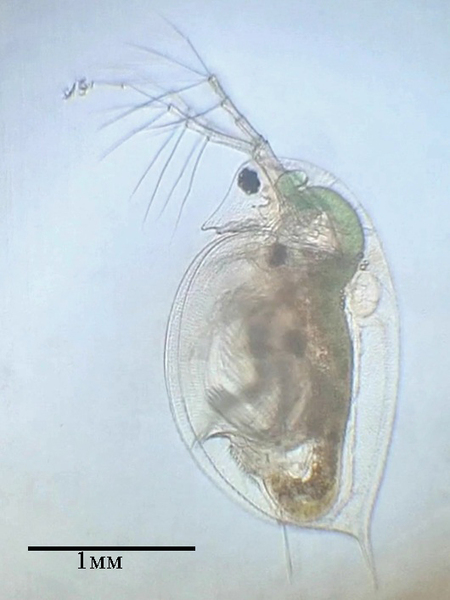
Chemical pollution can have significant effects on freshwater organisms. In this study, the effect of copper sulfate on the survival of Daphnia pulex and Ostracoda was investigated.
Read More...Formation and sticking of air bubbles in water in d-block containers
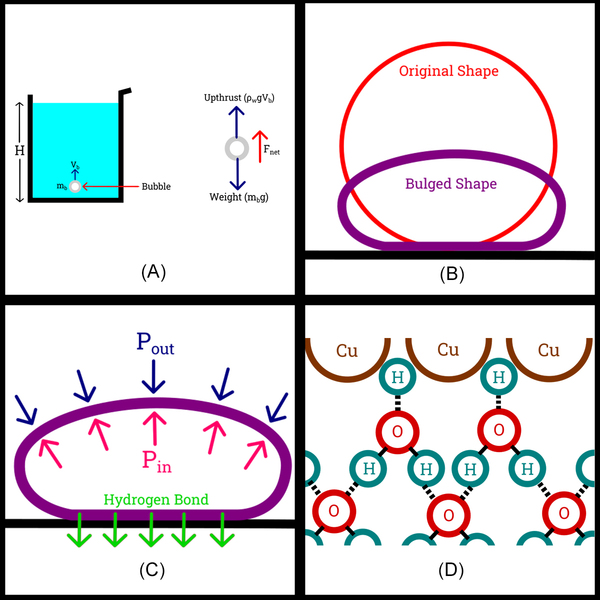
Bubbles! In this study, the authors investigate the effects that different materials, temperature, and distance have on the formation of water bubbles on the surface of copper and steel. They calculated mathematical relations based on the outcomes to better understand whether interstitial hydrogen present in the d-block metals form hydrogen bonds with the water bubbles to account for the structural and mechanical stability.
Read More...