
This study investigates the presence of alkaloids in a variety of medicinal plants using the Marquis reagent. They reveal some surprising results and how useful the Marquis reagent is.
Read More...Alkaloids Detection in Commonly Found Medicinal Plants with Marquis Reagent

This study investigates the presence of alkaloids in a variety of medicinal plants using the Marquis reagent. They reveal some surprising results and how useful the Marquis reagent is.
Read More...Modular mimics of neuroactive alkaloids - design, synthesis, and cholinesterase inhibitory activity of rivastigmine analogs
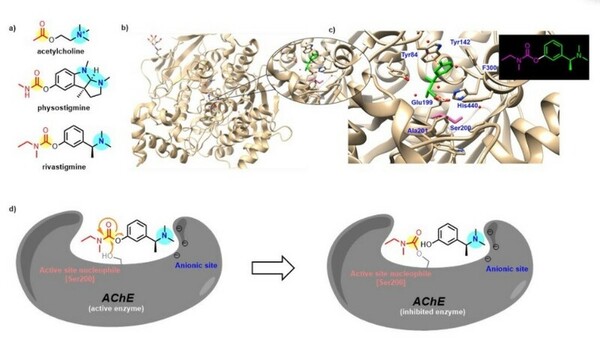
Naturally occurring neuroactive alkaloids are often studied for their potential to treat Neurological diseases. This team of students study Rivastigmine, a potent cholinesterase inhibitor that is a synthetic analog of physostigmine, which comes from the Calabar bean plant Physostigma venenosum. By comparing the effects of optimized synthetic analogs to the naturally occurring alkaloid, they determine the most favorable analog for inhibition of acetylcholinesterase (AChE), the enzyme that breaks down the neurotransmitter acetylcholine (ACh) to terminate neuronal transmission and signaling between synapses.
Read More...Phytochemical Analysis of Amaranthus spinosus Linn.: An in vitro Analysis
.png)
Mainstream cancer treatments, which include radiotherapy and chemotherapeutic drugs, are known to induce oxidative damage to healthy somatic cells due to the liberation of harmful free radicals. In order to avert this, physiological antioxidants must be complemented with external antioxidants. Here the authors performed a preliminary phytochemical screen to identify alkaloids, saponins, flavonoids, polyphenols, and tannins in all parts of the Amaranthus spinosus Linn. plant. This paper describes the preparation of this crude extract and assesses its antioxidant properties for potential use in complementary cancer treatment.
Read More...The anticancer and anti-inflammatory effects of polyherbal drug AS20 on HeLa cells resistant to 5-Fluorouracil
%20final%202-5-23.jpg)
The authors looked at 5-FU resistant HeLa cells and the ability of an herbal extract to show anti-inflammatory properties.
Read More...Apoptosis induction and anti-inflammatory activity of polyherbal drug AS20 on cervical cancer cell lines
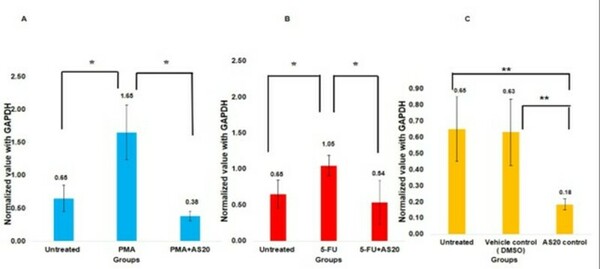
The authors found that treatment with AS20 suppressed phorbol 12-myristate 13-acetate (PMA) and 5-flurouracil (5-FU) induction of COX2 expression. We also observed AS20 treated cells showed DNA fragmentation in HeLa cells.
Read More...Cytotoxicity evaluation of Amaranthus extracts compared with AS20 on MCF-7 cancer cells
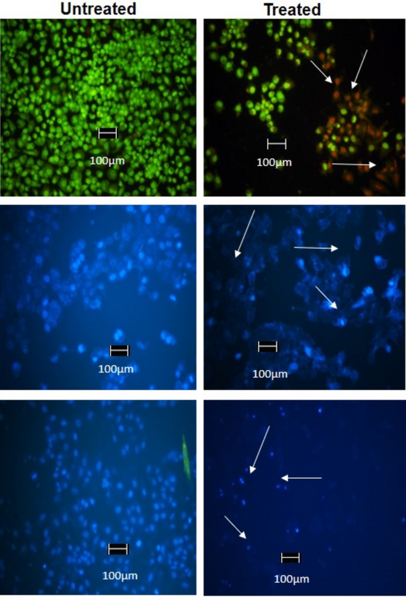
The authors test the antiproliferative and apoptosis-inducing properties of an extract created from a traditional Indian medicinal plant of the Amaranthus genus.
Read More...Formulation of novel polyherbal compound MAT20 with phytochemicals found in amla, tulsi, and moringa

With herbal plants providing an address to the adverse effects of oxidative stress found within the body, the authors of this article develop and assess a novel compound (“MAT20”) that blends three herbal plants for optimal oxidative stress relief.
Read More...Computational Structure-Activity Relationship (SAR) of Berberine Analogs in Double-Stranded and G-Quadruplex DNA Binding Reveals Both Position and Target Dependence
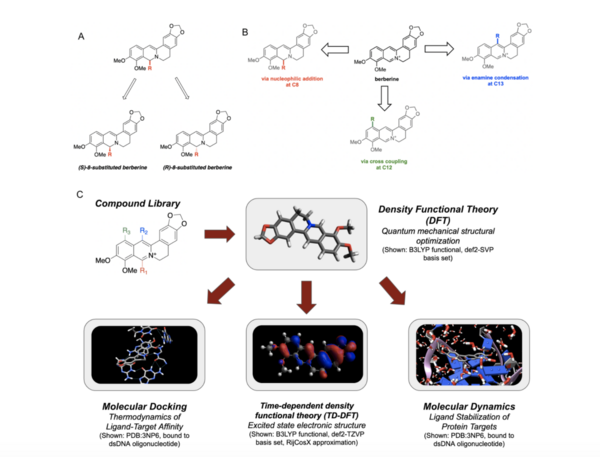
Berberine, a natural product alkaloid, and its analogs have a wide range of medicinal properties, including antibacterial and anticancer effects. Here, the authors explored a library of alkyl or aryl berberine analogs to probe binding to double-stranded and G-quadruplex DNA. They determined that the nature of the substituent, the position of the substituent, and the nucleic acid target affect the free energy of binding of berberine analogs to DNA and G-quadruplex DNA, however berberine analogs did not result in net stabilization of G-quadruplex DNA.
Read More...Structure-activity relationship of berberine and G4 DNA reveals aromaticity’s effect on binding affinity
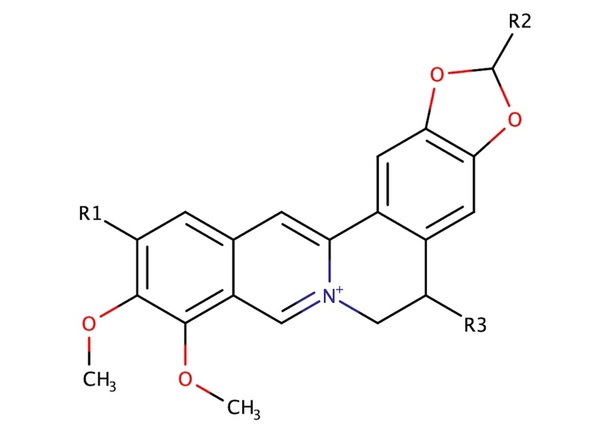
Berberine is a natural quaternary alkaloid that has anti-microbial and anti-cancer effects. This compound can bind to Guanine Quadruplex (G4) DNA secondary complexes to help inhibit cancer cell proliferation. In this study, the authors investigate whether incorporating large aromatic rings helps to stabilize berberine-G4 interactions.
Read More...Singlet oxygen production analysis of reduced berberine analogs via NMR spectroscopy
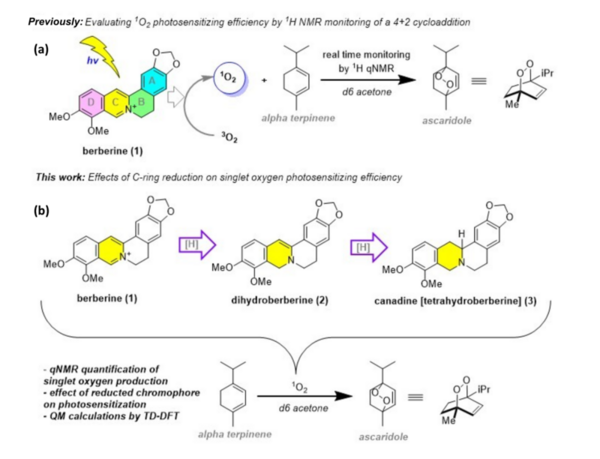
Berberine is a natural product isoquinoline alkaloid derived from plants of the genus Berberis. When exposed to photoirradiation, it produces singlet oxygen through photosensitization of triplet oxygen. Through qNMR analysis of 1H NMR spectra gathered through kinetic experiments, we were able to track the generation of a product between singlet oxygen and alpha terpinene, allowing us to quantitatively measure the photosensitizing properties of our scaffolds.
Read More...