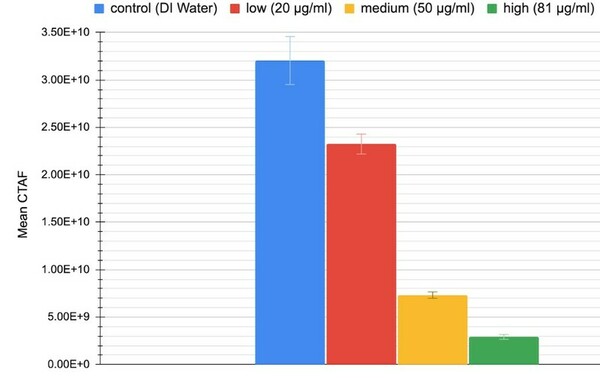
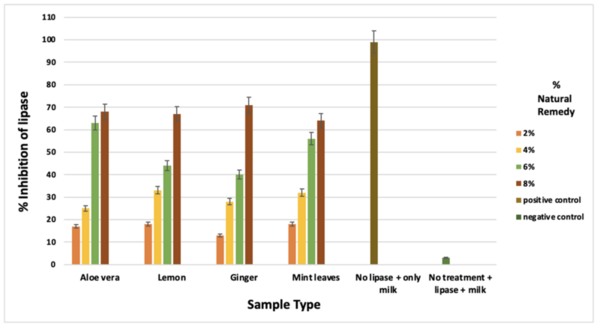
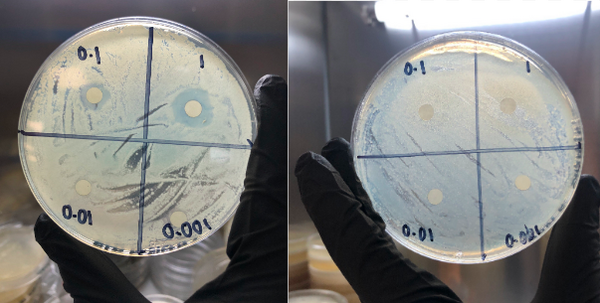
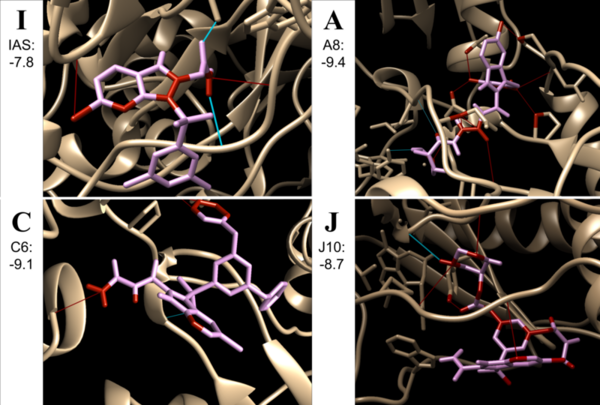
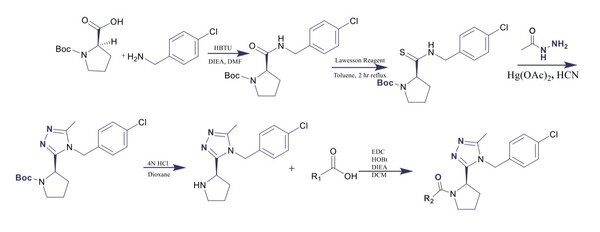
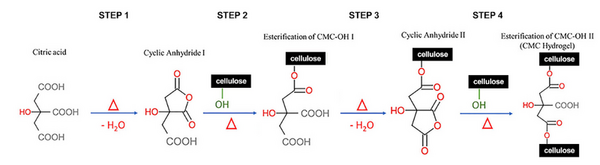
The goal of this study was to determine the if carbohydrates or complex carbohydrates are better for athlete's performance in anaerobic and aerobic exercise. Ultimately, we found that, when one’s schedule only allows for 30 minutes to eat before a workout, the best pre-workout meal for optimal glycogen levels to prompt muscle hypertrophy, strength increases, and better endurance is one that is simple carbohydrate-heavy.
Read More...