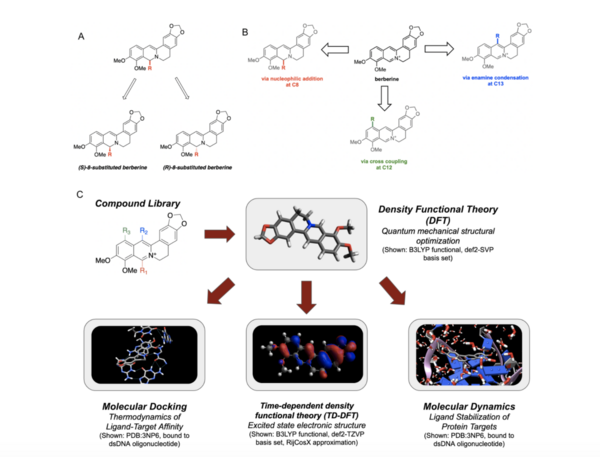
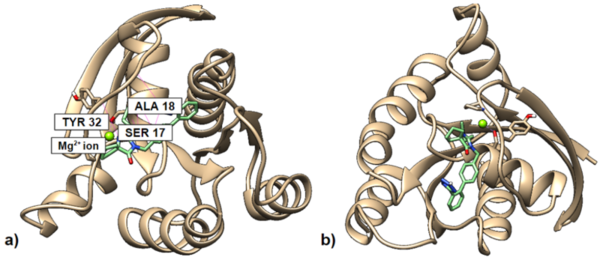
The Wnt signaling pathway, known to coordinate important aspects of cellular homeostasis ranging from differentiation, proliferation, migration, and much more, is dysregulated in many human diseases. This study demonstrates that aminomethylphosphonic acid, which is the main metabolite found in the common herbicide Glyphosate, is toxic to planaria and capable of binding to canonical Wnt proteins.
Read More...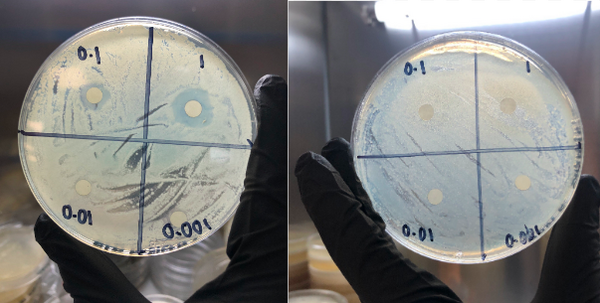
.jpeg)