The design of Benzimidazole derivatives to bind to GDP-bound K-RAS for targeted cancer therapy
(1) Aspiring Scholars Directed Research Program
https://doi.org/10.59720/23-166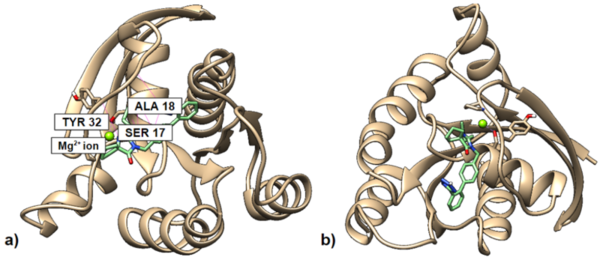
KRAS is a proto-oncogene that plays an important role in cell proliferation and division. It is found to be mutated in approximately 25% of cancer tumors. Several studies have tried to find ways to inhibit the KRAS protein, with modest success. This research aims to design molecules to bind to GDP-bound KRAS to predict how different molecules might bind to an inactivated version of the protein. We observed the binding affinity of various small molecules using UCSF Chimera and Autodock Vina and modified them to enhance their affinity to GDP-bound KRAS. We hypothesized that oxygen additions to the compound would increase hydrogen bonds, which in turn would increase the thermodynamic favorability of binding. We observed that molecules with favorable free-energy binding affinities tended to have more hydrogen bonds between the ligand and the protein, and they were mostly between oxygens in the ligand and various residues on the protein. Certain benzimidazole derivatives, such as Methiazole and Fenbendazole, have been found to inhibit growth of KRAS-mutant cells, so we tested several benzimidazole derivatives on GDP-bound KRAS in silico. Irbesartan had the most favorable binding affinity after modifications as compared to the other compounds docked on GDP-bound KRAS. The modified versions of Irbesartan can bind to GDP-bound KRAS better than the original Irbesartan.
This article has been tagged with: