Modeling stearoyl-coenzyme A desaturase 1 inhibitors to ameliorate α-Syn cytotoxicity in Parkinson's disease
(1) Los Altos High School
https://doi.org/10.59720/23-151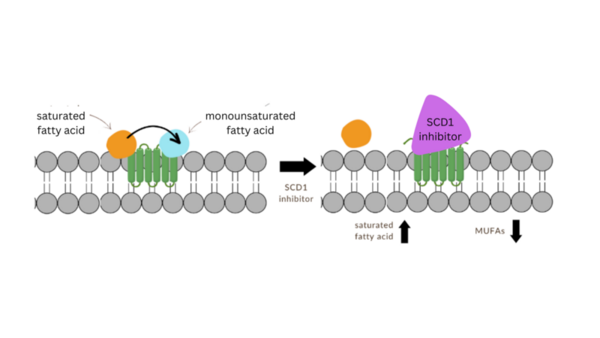
Parkinson’s disease is a form of progressive neurodegeneration that primarily affects dopaminergic neurons. It is characterized by misfolded α-Synuclein (α-Syn) proteins clumped together in Lewy bodies. More recently, it has been proposed that α-Syn toxicity may increase during interactions with fatty acids. There have been several studies linking stearoyl-coenzyme A desaturase 1 (SCD1), the rate-limiting enzyme for the conversion of saturated fatty acids (SFAs) to monounsaturated fatty acids (MUFAs), to the increased toxicity of α-Syn. Consequently, SCD1 inhibition is shown to decrease the toxicity and aggregation of α-Syn. However, the precise interactions of SCD1 inhibitors and SCD1 are unclear. This project compared seven novel analogs of SCD1 inhibitors, which we hypothesized to compete with SCD1’s coenzyme stearoyl coenzyme A, decreasing SFA conversion into their respective MUFAs. The analogs shared the same general pharmacophore with varying R groups (p-toluoyl, 4-fluorobenzoyl, 3-trifluoromethyl benzoyl, o-anisoyl, 3,4-difluorobenzoyl, 2-trifluoromethyl benzoyl, and 2-chlorobenzoyl). We hypothesized that analogs with the least steric hindrance would perform best. We drew a structure-activity relationship from in silico studies, with molecular docking results showing that four analogs were just as or more effective than MF-438, a commercially available SCD1 inhibitor. These results imply that the most effective R group was least sterically hindered, guiding further analog development in the field of small molecule Parkinson’s disease cures.
This article has been tagged with: