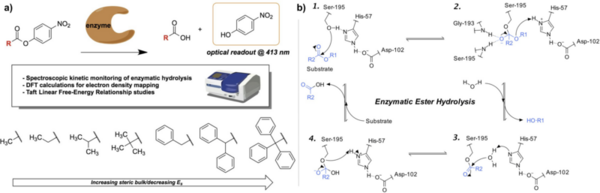
This study applies Taft linear free-energy relationships to study kinetic trends in the enzymatic hydrolysis of sterically hindered substrates.
Read More...Taft linear free-energy relationships in the biocatalytic hydrolysis of sterically hindered nitrophenyl ester substrates

This study applies Taft linear free-energy relationships to study kinetic trends in the enzymatic hydrolysis of sterically hindered substrates.
Read More...Monitoring the formation of polyurethane foams with an infrared camera: Classroom activity
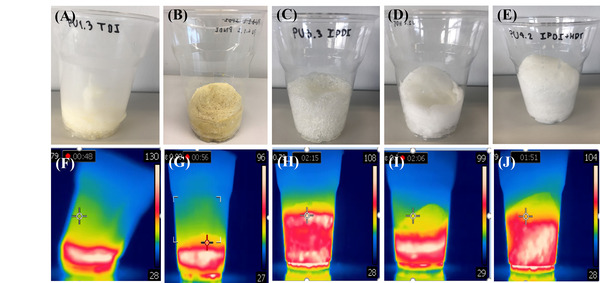
In this study, the authors utilize an infrared camera to visualize and investigate the exothermic reaction of polyurethane foam, which has many everyday uses including automotive seats, bedding, and insulation.
Read More...Breaking the Ice: A Scientific Take on the Ice Melting Abilities of Household Salts
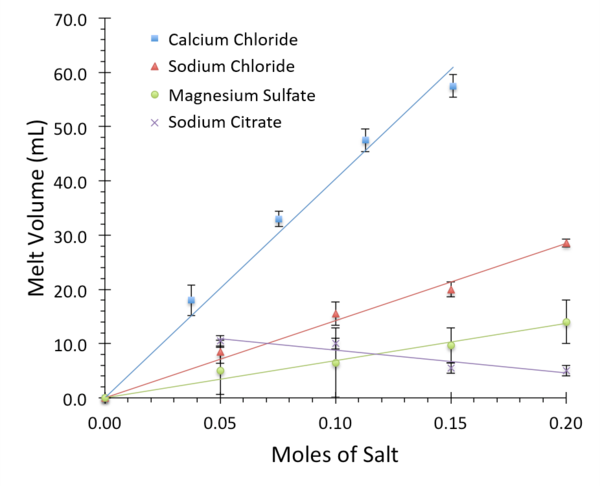
The use of salt to melt ice is a common and important practice to keep roadways safe during winter months. However, various subtypes of salt differ in their chemical and physical properties, as well as their environmental impact. In this study, the authors measure the effectiveness of different salts at disrupting ice structures and identify calcium chloride as the most effective.
Read More...Different volumes of acetic acid affect the oxygen production of spinach leaves during photosynthesis

The burning of fossil fuels, leading to an increased amount of carbon emissions, is the main cause of acid rain. Acid rain affects the process of photosynthesis, which makes the topic valuable to investigate. Our group utilizes plants to further investigate the relationship between pH value and photosynthesis. In this experiment, our group hypothesized that rain with a lower pH will decrease the rate of photosynthesis, causing less oxygen to be produced in the reaction.
Read More...Differentiating characteristics in exoplanet host stars
The authors looked at what conditions with host stars favor development of exoplanets.
Read More...The effect of circumference on the segregation of objects in a mixture
The authors test how the size-segregation theory applies to the behavior of hollow and irregular-shaped objects.
Read More...A comparison of starches and plasticizers for biopolymer synthesis and degradation
Nonthermal nitrogen fixation with air and water by using a low-pressure plasma
Optimizing surface contact area and electrolyte type to develop a more effective rechargeable battery
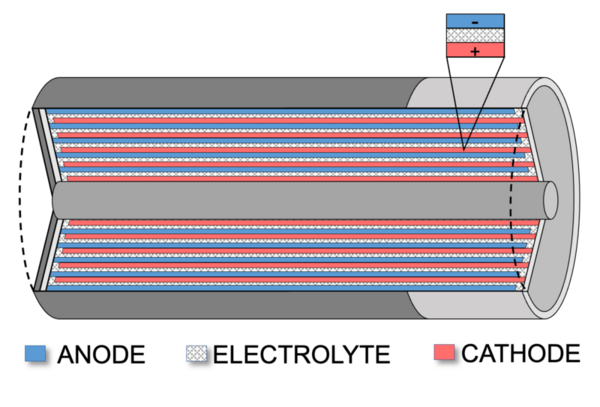
Rechargeable batteries are playing an increasingly prominent role in our lives due to the ongoing transition from fossil energy sources to green energy. The purpose of this study was to investigate variables that impact the effectiveness of rechargeable batteries. Alkaline (non-rechargeable) and rechargeable batteries share common features that are critical for the operation of a battery. The positive and negative electrodes, also known as the cathode and anode, are where the energy of the battery is stored. The electrolyte is what facilitates the transfer of cations and anions in a battery to generate electricity. Due to the importance of these components, we felt that a systematic investigation examining the surface area of the cathode and anode as well the impact of electrolytes with different properties on battery performance was justified. Utilizing a copper cathode and aluminum anode coupled with a water in salt electrolyte, a model rechargeable battery system was developed to test two hypotheses: a) increasing the contact area between the electrodes and electrolyte would improve battery capacity, and b) more soluble salt-based electrolytes would improve battery capacity. After soaking in an electrolyte solution, the battery was charged and the capacity, starting voltage, and ending voltage of each battery were measured. The results of this study supported our hypothesis that larger anode/cathodes surface areas and more ionic electrolytes such as sodium chloride, potassium chloride and potassium sulfate resulted in superior battery capacity. Incorporating these findings can help maximize the efficiency of commercial rechargeable batteries.
Read More...Converting SiO2 wafers to hydrophobic using chlorotrimethylsilane
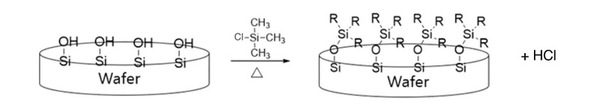
Semiconductors are the center of the fourth industrial revolution as they are key components for all electronics. Exposed wafers made of silicon (Si), which can easily oxidize, convert to silicon dioxide (SiO2). The surface of SiO2 wafers consists of many Si-OH bonds, allowing them to easily bond with water, resulting in a “wet” or hydrophilic condition. We sought to determine a way to modify the surface of SiO2 wafers to become hydrophobic to ensure safe wet cleaning.
Read More...