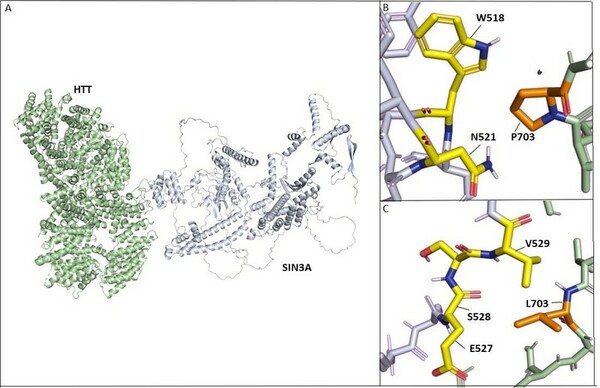
In an extensive study of gene mutations, and their resulting effect on protein-protein interactions, Desai and Stork found that HTT-PRPF40B-MECP2 interactions are weakened with progression of Lopes-Maciel-Rodan syndrome.
Read More...Disruptions in protein-protein interactions between HTT, PRPF40B, and MECP2 are involved in Lopes-Maciel-Rodan syndrome

In an extensive study of gene mutations, and their resulting effect on protein-protein interactions, Desai and Stork found that HTT-PRPF40B-MECP2 interactions are weakened with progression of Lopes-Maciel-Rodan syndrome.
Read More...Association of depression and suicidal ideation among adults with the use of H2 antagonists
In this study, the authors investigate associations between use of histamine H2 receptor antagonists and suicidal ideation in different age groups.
Read More...A potentially underestimated source of CO2 and other greenhouse gases in agriculture

Here the authors investigated the role of agricultural fertilizers as potential contributors to greenhouse gas emissions. In contrast to the typical investigations that consider microbiological processes, the authors considered purely chemical processes. Based on their results they found that as much as 20.41% of all CO2 emission from land-based activities could be a result of mineral nitrogen fertilizers.
Read More...Modelling effects of alkylamines on sea salt aerosols using the Extended Aerosols and Inorganics Model

With monitoring of climate change and the evolving properties of the atmosphere more critical than ever, the authors of this study take sea salt aerosols into consideration. These sea salt aerosols, sourced from the bubbles found at the surface of the sea, serve as cloud condensation nuclei (CCN) and are effective for the formation of clouds, light scattering in the atmosphere, and cooling of the climate. With amines being involved in the process of CCN formation, the authors explore the effects of alkylamines on the properties of sea salt aerosols and their potential relevance to climate change.
Read More...Evaluation of platelet-rich plasma vs. platelet lysate: VEGF and PDGF concentration, stability, and shelf life

Cell-free biologicals are a novel method of treating clinical conditions which involve chronic inflammation such as tendonitis and osteoarthritis. This study compared platelet-derived growth factor (PDGF) and vascular endothelial growth factor (VEGF) in platelet-rich plasma (PRP), activated PRP (aPRP), and platelet lysate (PL). It was hypothesized that PL would contain higher concentrations of growth factors than PRP and that different storage temperatures for PL would diminish cytokine expression. Results demonstrated PL had the highest concentrations of both cytokines, with concentrations slightly diminishing at-80C. aPRP and PRP demonstrated lower concentrations of PDGF and VEGF than PL.
Read More...Preliminary investigation of Allosauroidea facial integument and the evolution of theropod facial armor
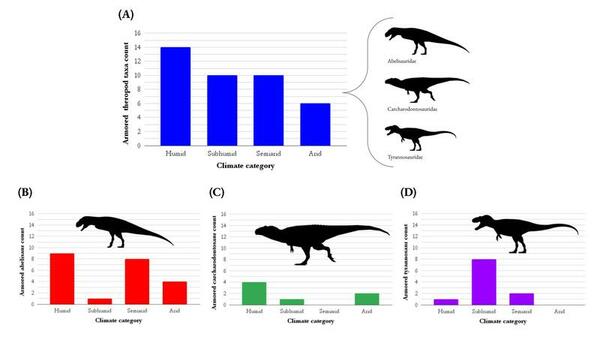
The facial integument, or external skin tissues, were assessed on set of dinosaurs from the Allosauroidea clade to test whether dermal patterns served specific functions.
Read More...Virtual Screening of Cutibacterium acnes Antibacterial Agent Using Natural Compounds Database
A common form of Acne is caused by a species of bacterium called Cutibacterium acnes. By using a predictive algorithm and structural analysis, the authors identified 5 small molecules with high affinity to growth factors in Catibacterium acnes. This has potential implications for supplemental skincare products.
Read More...Eggshell consumption in different reproductive stages and broods of the Western Bluebird, Sialia mexicana

The authors investigate whether Western Bluebirds and other perching birds consume eggshells, as a source of calcium, at a greater rate before reproduction and during nest building when they are unable to store calcium.
Read More...Investigating Hydrogen as a Potential Alternative to Kerosene in Fueling Commercial Aircraft
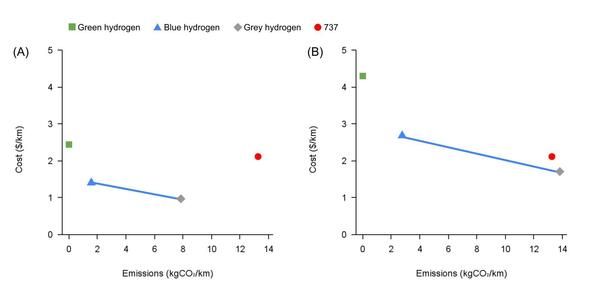
Growing climate concerns have intensified research into zero-emission transportation fuels, notably hydrogen. Hydrogen is considered a clean fuel because its only major by-product is water. This project analyzes how hydrogen compares to kerosene as a commercial aircraft fuel with respect to cost, CO2 emissions, and flight range.
Read More...PCR technology for screening genetically modified soybeans

In order to determine whether unmarked soybeans in the market were genetically modified crops, the authors developed a polymerase chain reaction (PCR) screen for DNA lectin.
Read More...