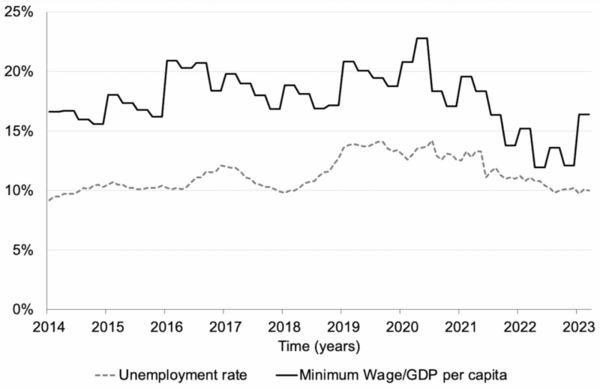
The authors looked at the relationship between unemployment and minimum wage in Turkey (Türkiye). They found that there is a positive correlation between minimum wage and unemployment.
Read More...Determining the relationship between unemployment and minimum wage in Turkey

The authors looked at the relationship between unemployment and minimum wage in Turkey (Türkiye). They found that there is a positive correlation between minimum wage and unemployment.
Read More...Impacts of the gut microbiota on arginine synthesis
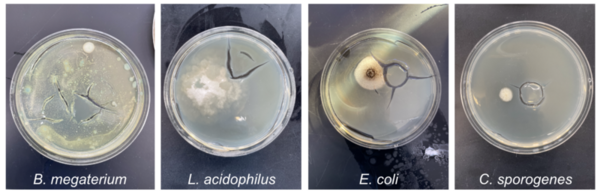
In this article the authors looked at arginine synthesis across different bacteria commonly found in different regional diets. They found that B. megaterium and C. sporogenes both caused a higher pH to occur on their agar plates compared to other bacteria tested indicating a greater amount of arginine synthesis.
Read More...Genetic algorithm based features selection for predicting the unemployment rate of India

The authors looked at using genetic algorithms to look at the Indian labor market and what features might best explain any variation seen. They found that features such as economic growth and household consumption, among others, best explained variation.
Read More...Inhibitory effects of captan on growth of Escherichia coli and Bacillus coagulans
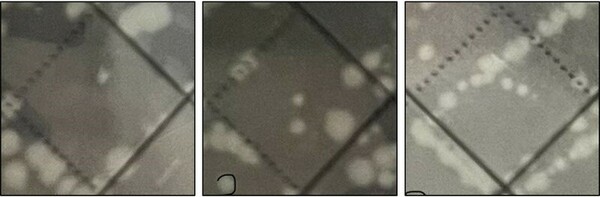
The authors test the effects of the pesticide captan on the growth of gut microbiome bacteria including Bacillus coagulan and Escherichia coli.
Read More...Increased carmine red exposure periods yields a higher number of vacuoles formed in Tetrahymena pyriformis
.jpeg)
T. pyriformis can use phagocytosis to create vacuoles of carmine red, a dye which is made using crushed insects and is full of nutrients. Establishing a relationship between vacuole formation and duration of exposure to food can demonstrate how phagocytosis occurs in T. pyriformis. We hypothesized that if T. pyriformis was incubated in a carmine red solution, then more vacuoles would form over time in each cell.
Read More...Eggshell consumption in different reproductive stages and broods of the Western Bluebird, Sialia mexicana

The authors investigate whether Western Bluebirds and other perching birds consume eggshells, as a source of calcium, at a greater rate before reproduction and during nest building when they are unable to store calcium.
Read More...Psychosocial impact of home-based learning among students during the COVID-19 Pandemic in Singapore

In this study, the authors surveyed a number of students in Singapore to determine how their experiences changed after the implementation of home-based learning during the COVID-19 pandemic.
Read More...Temperatures of 20°C Produce Increased Net Primary Production in Chlorella sp.
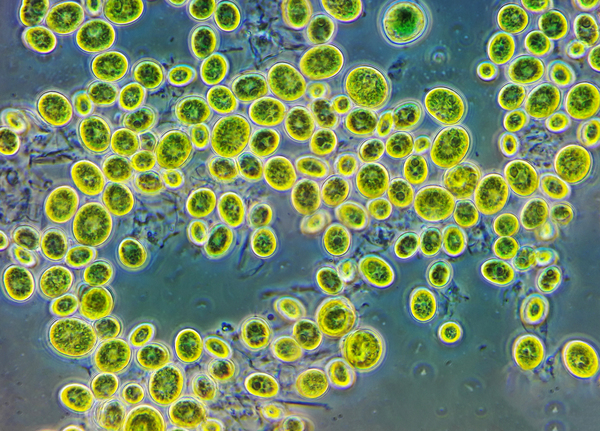
Chlorella sp. are unicellular green algae that use photosynthesis to reduce carbon dioxide into glucose. In this study, authors sought to determine the temperature that Chlorella sp. is maximally efficient at photosynthesis, and therefore removing the most carbon dioxide from the system. This activity could be harnessed to naturally remove carbon dioxide from the environment, fighting the effects of climate change.
Read More...Biowaste to Biofuel: Using Methane-Producing Microorganisms Found in Soil Samples from Local Wetlands
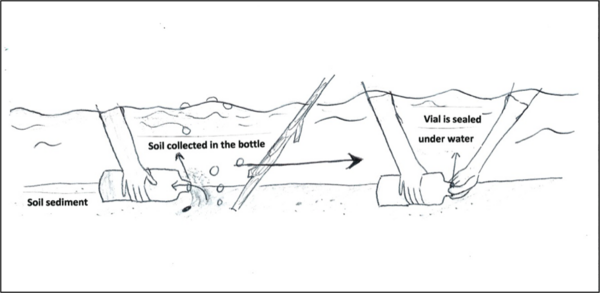
Methane is a naturally-occurring gas that could be utilized as a renewable source of energy. In this study, authors isolated microorganisms from the Puget Sound region that could produce methane biofuel from composted waste.
Read More...Hydrogen Sulfide Inhibits Flowering but Hastens New Leaf Growth in Bok Choy (Chinese Cabbage)
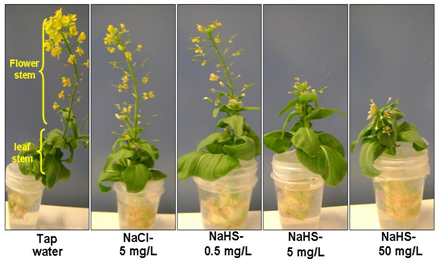
Hydrogen sulfide is toxic at high concentrations, but at low concentrations may be helpful for plant growth. This study characterizes the effect of hydrogen sulfide exposure on leafy plant growth. Bok choy hearts were grown in the presence of hydrogen sulfide, and measured for new leaf growth and flowering.
Read More...