Quantitative NMR spectroscopy reveals solvent effects in the photochemical degradation of thymoquinone
(1) Department of Chemistry, Biochemistry, & Physics, Aspiring Scholars Directed Research Program
https://doi.org/10.59720/22-116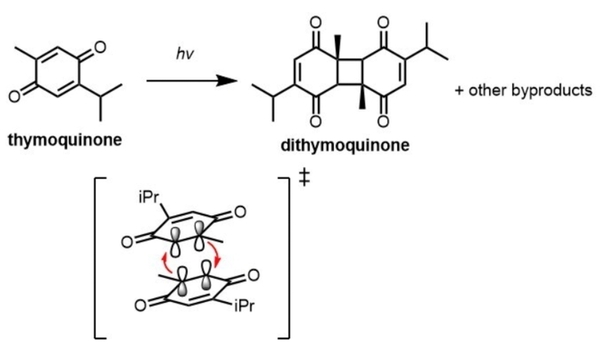
Thymoquinone, a monoterpene natural product isolated from Nigella sativa, has demonstrated potent antioxidant, cytotoxic, anticancer, and anti-inflammatory activity, making it a compound of great therapeutic potential and scientific interest. However, as a photochemically labile compound that can dimerize or decompose into other compounds upon irradiation, its clinical administration and synthetic modifications are greatly limited by its instability in the presence of light. Therefore, it is crucial to have a reliable way to quantify decreases in thymoquinone concentration as an indicator of photodegradation to provide insight into the chemical use of the compound. Here, we employed quantitative 1H nuclear magnetic resonance (NMR) spectroscopy as well as computational time-dependent density functional theory (TD-DFT) calculations to identify the effect of solvation on the photochemical degradation of thymoquinone under ultraviolet light (UV). We found that the rate of photochemical degradation of thymoquinone is highly solvent dependent, and that greatest photodegradation occurs in chloroform. The results presented here are informative for future solvent selection related to the isolation and experimental usage of the compound in solution.
This article has been tagged with: