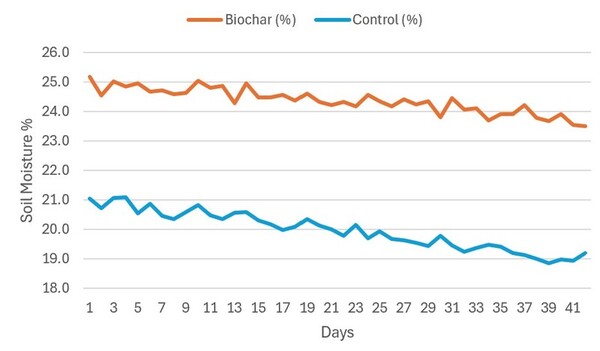
The study explored converting Gracilaria seaweed waste—known for releasing toxic hydrogen sulfide when decomposed—into biochar as a sustainable solution for waste management and soil improvement.
Read More...Enhanced soil fertility through seaweed-derived biochar: A comparative analysis with commercial fertilizers

The study explored converting Gracilaria seaweed waste—known for releasing toxic hydrogen sulfide when decomposed—into biochar as a sustainable solution for waste management and soil improvement.
Read More...A systematic study of cut-resistant socks for hockey players
Improving Wound Healing by Breaking Down Biofilm Formation and Reducing Nosocomial Infections
In a 10-year period in the early 2000’s, hospital-based (nosocomial) infections increased by 123%, and this number is increasing as time goes on. The purpose of this experiment was to use hyaluronic acid, silver nanoparticles, and a bacteriophage cocktail to create a hydrogel that promotes wound healing by increasing cell proliferation while simultaneously disrupting biofilm formation and breaking down Staphylococcus aureus and Pseudomonas aeruginosa, which are two strains of bacteria that attribute to nosocomial infections and are increasing in antibiotic resistance.
Read More...Hydrogen Sulfide Inhibits Flowering but Hastens New Leaf Growth in Bok Choy (Chinese Cabbage)
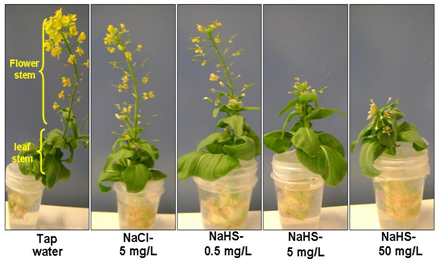
Hydrogen sulfide is toxic at high concentrations, but at low concentrations may be helpful for plant growth. This study characterizes the effect of hydrogen sulfide exposure on leafy plant growth. Bok choy hearts were grown in the presence of hydrogen sulfide, and measured for new leaf growth and flowering.
Read More...Investigating Hydrogen as a Potential Alternative to Kerosene in Fueling Commercial Aircraft
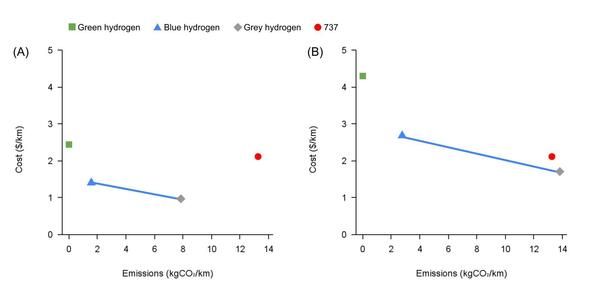
Growing climate concerns have intensified research into zero-emission transportation fuels, notably hydrogen. Hydrogen is considered a clean fuel because its only major by-product is water. This project analyzes how hydrogen compares to kerosene as a commercial aircraft fuel with respect to cost, CO2 emissions, and flight range.
Read More...The Effect of Antioxidant Vitamins on Mustard Plants in a Hydrogen Peroxide-Induced Injury Model

In this study, the authors assess the antioxidant properties of vitamins A, C and E given to mustard plants after oxidative damage. This research shows an interesting comparison of the vitamins' effect on plant recovery and health.
Read More...Yeast catalysis of hydrogen peroxide as an enhanced chemical treatment method for harvested rainwater
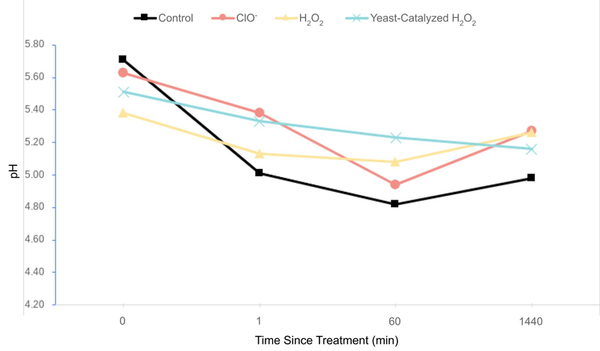
The authors looked at different treatments to clean up rainwater collected at home. They found that chlorine treatment and treatment with hydrogen peroxide catalyzed by yeast showed similar potential for cleaning up contaminated rainwater, but that further studies are needed to better assess impact on specific contaminant levels still present.
Read More...How Ethanol Concentration Affects Catalase Catalysis of Hydrogen Peroxide
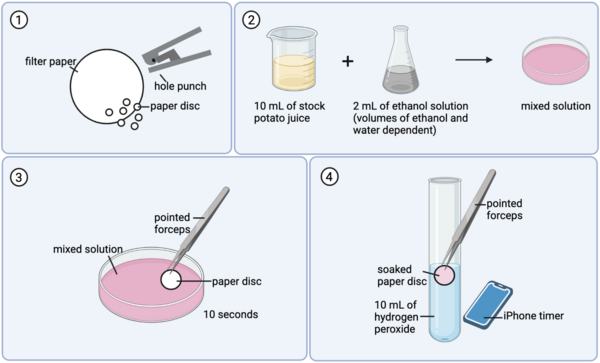
Catalase is a critical enzyme in the human body because it is capable of converting potentially dangerous hydrogen peroxide into water and oxygen. This work asks whether ethanol affects catalase activity, as alcohol consumption has been often linked to hepatitis occurring in the liver, where catalase level is especially high, and ethanol is known to be capable of denaturing proteins. Testing different concentrations of ethanol found that higher concentrations reduced the activity of catalase. This work has important implications on the negative effects of ethanol on metabolism, in which catalase plays an important role, and protein function more broadly.
Read More...Racemic serine is less soluble than pure enantiomers due to stronger intermolecular hydrogen bonds
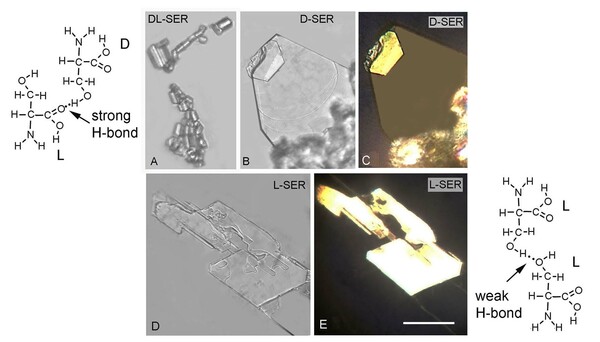
Seeking to develop a better understanding of the chemical and physical properties of amino acids that compose proteins, here the authors investigated the unusual relative insolubility of racemic mixtures of D- and L-serine compared to the solubility of pure D- or L-serine. The authors used a combination of microscopy and temperature measurements alongside previous X-ray diffraction studies to conclude that racemic DL-serine crystals consist of comparatively stronger hydrogen bond interactions compared to crystals of pure enantiomers. These stronger interactions were found to result in the unique release of heat during the crystallization of racemic mixtures.
Read More...The Protective Antioxidant Effects of Sulforaphane on Germinating Radish Seeds Treated with Hydrogen Peroxide
Free radical chain reactions result when atoms containing unpaired electrons bind with biomolecules and alter their biological functions, contributing to the progression of diseases such as atherosclerosis, cancer, and diabetes. Antioxidants, such as vitamin E and sulforaphane, are effective neutralizers of free radicals and prevent cellular damage. This present study is conducted to determine the relative effectiveness of sulforaphane against free radicals generated by hydrogen peroxide (H2O2) compared with the known antioxidant vitamin E.
Read More...