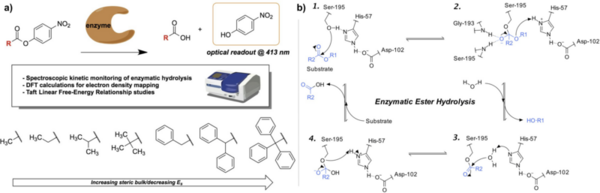
This study applies Taft linear free-energy relationships to study kinetic trends in the enzymatic hydrolysis of sterically hindered substrates.
Read More...Taft linear free-energy relationships in the biocatalytic hydrolysis of sterically hindered nitrophenyl ester substrates

This study applies Taft linear free-energy relationships to study kinetic trends in the enzymatic hydrolysis of sterically hindered substrates.
Read More...Modeling stearoyl-coenzyme A desaturase 1 inhibitors to ameliorate α-Syn cytotoxicity in Parkinson's disease
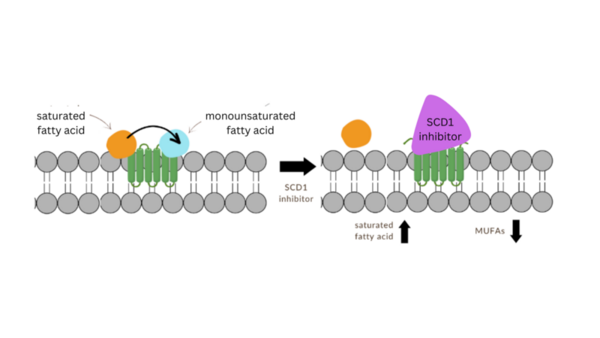
The authors use molecular modeling to test analogs of the stearoyl-coenzyme A desaturase 1 (SCD1) inhibitor MF-438 with implications for future development of Parkinson's disease therapeutics.
Read More...Association of depression and suicidal ideation among adults with the use of H2 antagonists
In this study, the authors investigate associations between use of histamine H2 receptor antagonists and suicidal ideation in different age groups.
Read More...