
The authors analyzed biosolids from five Wisconsin wastewater treatment plants and suggest using KBr pellet FTIR as a simple and rapid method to start characterizing P species in biosolids.
Read More...Fourier-Transform Infrared (FTIR) spectroscopy analysis of seven wisconsin biosolids

The authors analyzed biosolids from five Wisconsin wastewater treatment plants and suggest using KBr pellet FTIR as a simple and rapid method to start characterizing P species in biosolids.
Read More...Utilizing a Wastewater-Based Medium for Engineered Saccharomyces cerevisiae for the Biological Production of Fatty Alcohols and Carboxylic Acids to Replace Petrochemicals
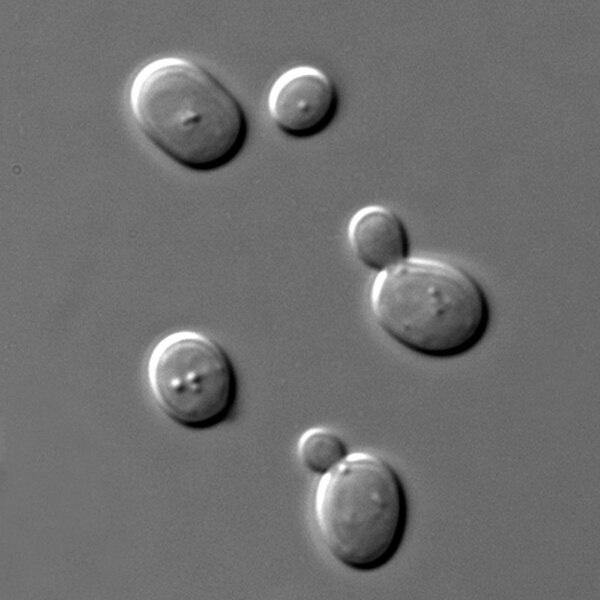
Saccharomyces cerevisiae yeast is used to produce bioethanol, an alternative to fossil fuels. In this study, authors take advantage of this well studied yeast by genetically engineering them to increase fatty acid biosynthesis and culturing in a cost-effective wastewater based medium; potentially providing a sustainable alternative to petrochemicals.
Read More...Developing a Method to Remove Inorganic Arsenic from Rice with Natural Substances

In this study, the authors tested different approaches for removing arsenic from rice. Due to higher arsenic levels in water, some areas grow rice with higher levels as well. This is a health hazard and so developing methods to remove arsenic from the rice will be helpful to many. Using a rapid arsenic kit, the authors found that activated charcoal was the most effective at removing arsenic from rice.
Read More...Mitigating microplastic exposure from water consumption in junior high students and teachers

Microplastics (MPs) are inorganic material that have been observed within items destined for human consumption, including water, and may pose a potential health hazard. Here we estimated the average amount of MPs junior high students and teachers consumed from different water sources and determined whether promoting awareness of microplastic (MP) exposure influenced choice of water source and potential MPs consumed.
Read More...Managing CO2 levels through precipitation-based capture from seawater and electrochemical conversion
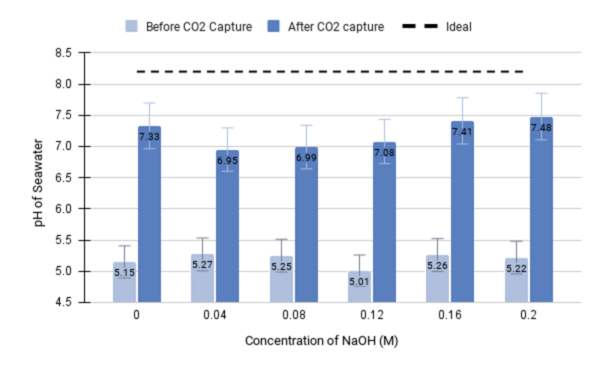
The authors set out to develop an electrochemical device that would have efficient and sustained carbon dioxide capture.
Read More...Taft linear free-energy relationships in the biocatalytic hydrolysis of sterically hindered nitrophenyl ester substrates
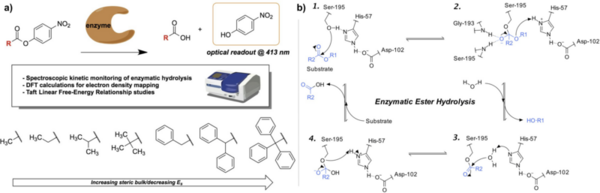
This study applies Taft linear free-energy relationships to study kinetic trends in the enzymatic hydrolysis of sterically hindered substrates.
Read More...Voltage, power, and energy production of a Shewanella oneidensis biofilm microbial fuel cell in microgravity
.PNG)
The authors looked at the ability of Shewanella oneidensis to generate energy in a microbial fuel cell under varying conditions. They found that the S. Onedensis biofilm was able to produce energy in microgravity and that one of the biggest factors that limited energy production was a decrease in growth medium present.
Read More...Modelling effects of alkylamines on sea salt aerosols using the Extended Aerosols and Inorganics Model

With monitoring of climate change and the evolving properties of the atmosphere more critical than ever, the authors of this study take sea salt aerosols into consideration. These sea salt aerosols, sourced from the bubbles found at the surface of the sea, serve as cloud condensation nuclei (CCN) and are effective for the formation of clouds, light scattering in the atmosphere, and cooling of the climate. With amines being involved in the process of CCN formation, the authors explore the effects of alkylamines on the properties of sea salt aerosols and their potential relevance to climate change.
Read More...Spectrophotometric comparison of 4-Nitrophenyl carbonates & carbamates as base-labile protecting groups
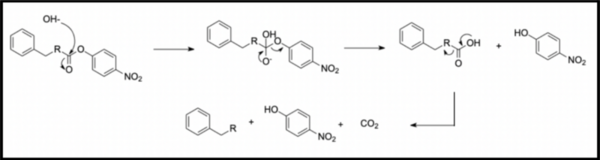
In organic synthesis, protecting groups are derivatives of reactive functionalities that play a key role in ensuring chemoselectivity of chemical transformations. To protect alcohols and amines, acid-labile tert-butyloxycarbonyl protecting groups are often employed but are avoided when the substrate is acid-sensitive. Thus, orthogonal base-labile protecting groups have been in demand to enable selective deprotection and to preserve the reactivity of acid-sensitive substrates. To meet this demand, we present 4-nitrophenyl carbonates and carbamates as orthogonal base-labile protecting group strategies.
Read More...Kinetic Monitoring and Fourier-Transform Infrared (FTIR) Spectroscopy of the Green Oxidation of (-)-Menthol to (-)-Menthone
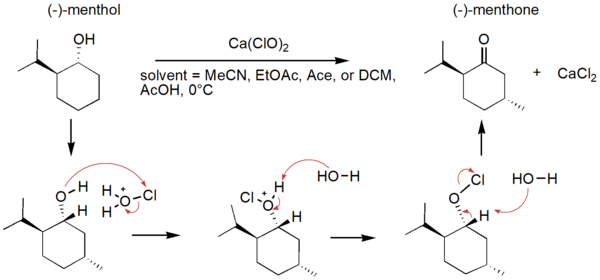
In an effort to reduce the production of hazardous substances, green chemistry aims to make chemical processes more sustainable. One way to do so is changing solvents in chemical reactions. Here, authors assessed different “green” solvents on the oxidation of (-)-menthol to (-)-menthone using Fourier-transform infrared (FTIR) spectroscopy, optimizing the solvent system for this reaction.
Read More...