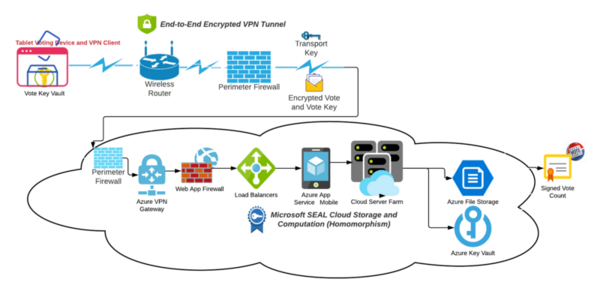
In this study, the authors present proposed cryptographic controls for election sites with the hypothesis that this will mitigate risk and remediate vulnerabilities.
Read More...Design and implementation of a cryptographically secure electronic voting infrastructure

In this study, the authors present proposed cryptographic controls for election sites with the hypothesis that this will mitigate risk and remediate vulnerabilities.
Read More...Design and in silico screening of analogs of rilpivirine as novel non-nucleoside reverse transcriptase inhibitors (NNRTIs) for antiretroviral therapy
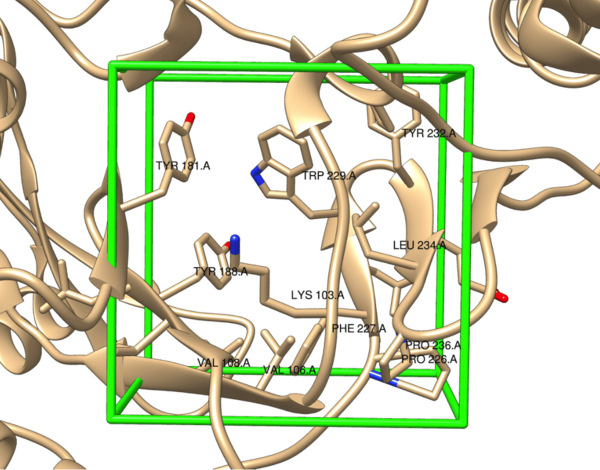
In this study, the authors use high-throughput virtual screening to design and evaluate a set of non-nucleoside reverse transcriptase inhibitors for binding affinity to the protein reverse transcriptase. These studies have important applications toward HIV therapies.
Read More...Designing gRNAs to reduce the expression of the DMPK gene in patients with classic myotonic dystrophy

The authors describe the design and testing of new guide RNAs targeting the DMPK gene, which is responsible for myotonic dystrophy.
Read More...AI-designed mini-protein targeting claudin-5 to enhance blood–brain barrier integrity
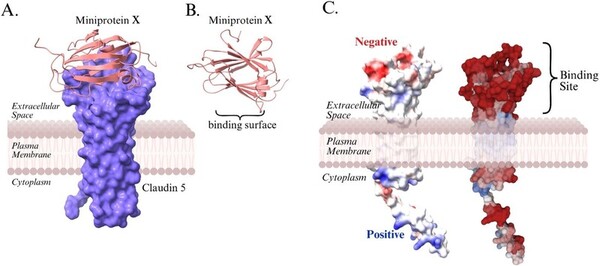
The authors employ computational protein design to identify a mini-protein with the potential to enhance binding of the tight junction protein, claudin-5, at the blood-blood barrier with therapeutic potential for neurodegenerative diseases.
Read More...The design of Benzimidazole derivatives to bind to GDP-bound K-RAS for targeted cancer therapy
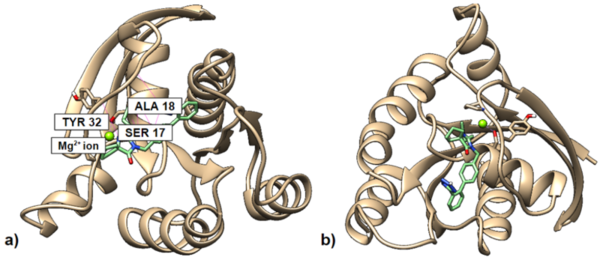
In this study, the authors looked at a proto-oncogene, KRAS, and searched for molecules that are predicted to be able to bind to the inactive form of KRAS. They found that a modified version of Irbesartan, a derivative of benzimidazole, showed the best binding to inactive KRAS.
Read More...In silico design of novel acetylcholinesterase inhibitors as potential therapeutics for Alzheimer's disease
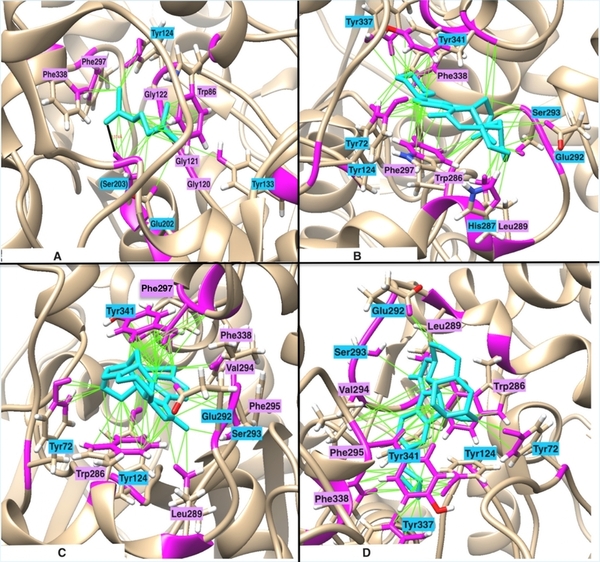
Elevated acetylcholinesterase (AChE) activity contributes to cognitive decline and neurodegenerative diseases such as Alzheimer’s, motivating the search for more effective inhibitors with better bioavailability. This study used computational methods to design novel, non-toxic AChE inhibitors.
Read More...Reactivity-informed design, synthesis, and Michael addition kinetics of C-ring andrographolide analogs
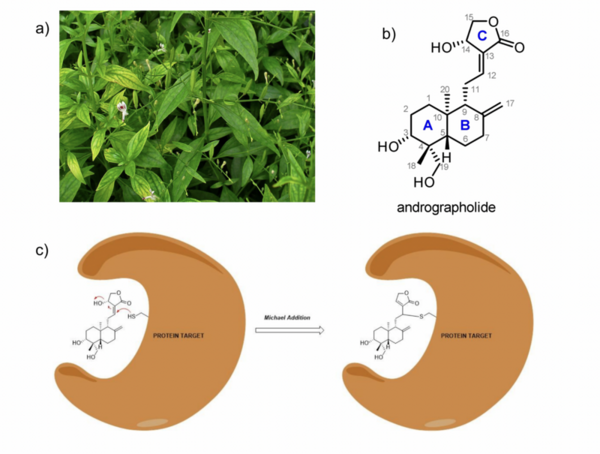
Here, based on the identification of androgapholide as a potential therapeutic treatment against cancer, Alzheimer's disease, diabetes, and multiple sclerosis, due to its ability to inhibit a signaling pathway in immune system function, the authors sought ways to optimize the natural product human systems by manipulating its chemical structure. Through the semisynthesis of a natural product along with computational studies, the authors developed an understanding of the kinetic mechanisms of andrographolide and semisynthetic analogs in the context of Michael additions.
Read More...Open Source RNN designed for text generation is capable of composing music similar to Baroque composers
Recurrent neural networks (RNNs) are useful for text generation since they can generate outputs in the context of previous ones. Baroque music and language are similar, as every word or note exists in context with others, and they both follow strict rules. The authors hypothesized that if we represent music in a text format, an RNN designed to generate language could train on it and create music structurally similar to Bach’s. They found that the music generated by our RNN shared a similar structure with Bach’s music in the input dataset, while Bachbot’s outputs are significantly different from this experiment’s outputs and thus are less similar to Bach’s repertoire compared to our algorithm.
Read More...Modular mimics of neuroactive alkaloids - design, synthesis, and cholinesterase inhibitory activity of rivastigmine analogs
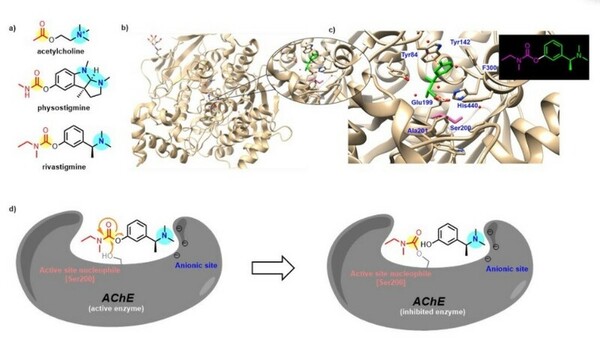
Naturally occurring neuroactive alkaloids are often studied for their potential to treat Neurological diseases. This team of students study Rivastigmine, a potent cholinesterase inhibitor that is a synthetic analog of physostigmine, which comes from the Calabar bean plant Physostigma venenosum. By comparing the effects of optimized synthetic analogs to the naturally occurring alkaloid, they determine the most favorable analog for inhibition of acetylcholinesterase (AChE), the enzyme that breaks down the neurotransmitter acetylcholine (ACh) to terminate neuronal transmission and signaling between synapses.
Read More...Redesigning an Experiment to Determine the Coefficient of Friction
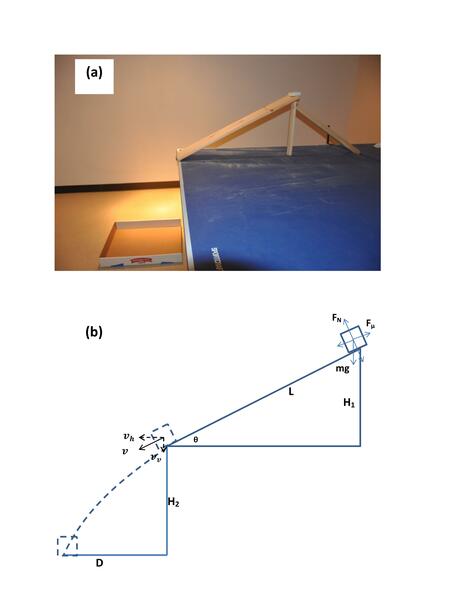
In a common high school experiment to measure friction coefficients, a weighted mass attached to a spring scale is dragged across a surface at a constant velocity. While the constant velocity is necessary for an accurate measurement, it can be difficult to maintain and this can lead to large errors. Here, the authors designed a new experiment to measure friction coefficients in the classroom using only static force and show that their method has a lower standard deviation than the traditional experiment.
Read More...