
In this study, the authors determine optimal pH levels for maximizing isopropanol degradation in water. This has important applications for cleaning up polluted wastewater in the environment.
Read More...Optimal pH for indirect electrochemical oxidation of isopropyl alcohol with Ru-Ti anode and NaCl electrolyte

In this study, the authors determine optimal pH levels for maximizing isopropanol degradation in water. This has important applications for cleaning up polluted wastewater in the environment.
Read More...Incorporating graphite from pencils as a component of lithium-ion batteries
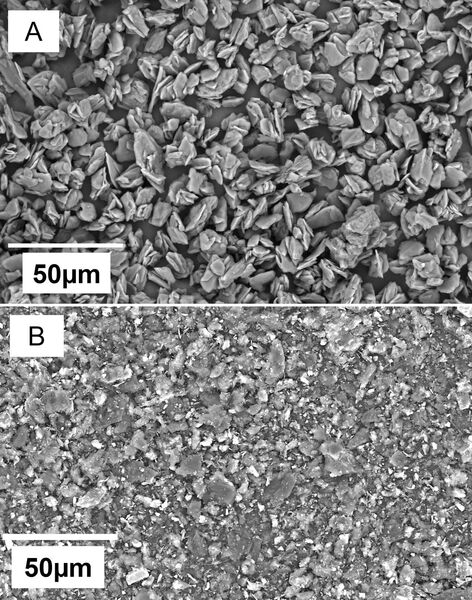
The authors looked at the ability to use graphite from pencils in anodes of lithium-anode batteries.
Read More...Optimizing surface contact area and electrolyte type to develop a more effective rechargeable battery
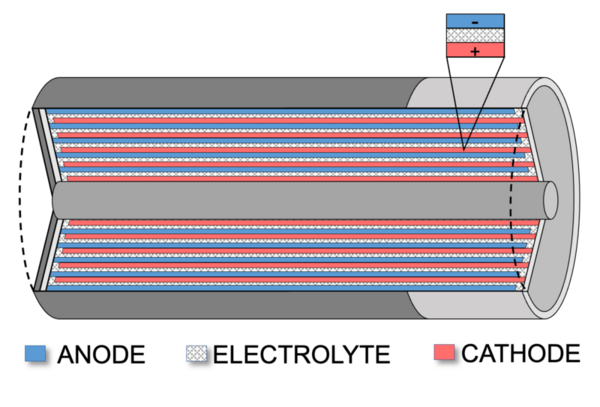
Rechargeable batteries are playing an increasingly prominent role in our lives due to the ongoing transition from fossil energy sources to green energy. The purpose of this study was to investigate variables that impact the effectiveness of rechargeable batteries. Alkaline (non-rechargeable) and rechargeable batteries share common features that are critical for the operation of a battery. The positive and negative electrodes, also known as the cathode and anode, are where the energy of the battery is stored. The electrolyte is what facilitates the transfer of cations and anions in a battery to generate electricity. Due to the importance of these components, we felt that a systematic investigation examining the surface area of the cathode and anode as well the impact of electrolytes with different properties on battery performance was justified. Utilizing a copper cathode and aluminum anode coupled with a water in salt electrolyte, a model rechargeable battery system was developed to test two hypotheses: a) increasing the contact area between the electrodes and electrolyte would improve battery capacity, and b) more soluble salt-based electrolytes would improve battery capacity. After soaking in an electrolyte solution, the battery was charged and the capacity, starting voltage, and ending voltage of each battery were measured. The results of this study supported our hypothesis that larger anode/cathodes surface areas and more ionic electrolytes such as sodium chloride, potassium chloride and potassium sulfate resulted in superior battery capacity. Incorporating these findings can help maximize the efficiency of commercial rechargeable batteries.
Read More...The Development and Maximization of a Novel Photosynthetic Microbial Fuel Cell Using Rhodospirillum rubrum
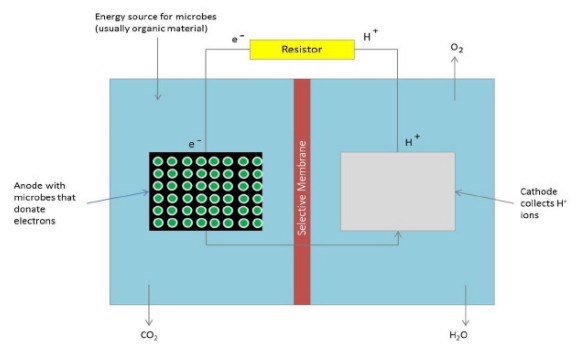
Microbial fuel cells (MFCs) are bio-electrochemical systems that utilize bacteria and are promising forms of alternative energy. Similar to chemical fuel cells, MFCs employ both an anode (accepts electrons) and a cathode (donates electrons), but in these devices the live bacteria donate the electrons necessary for current. In this study, the authors assess the functionality of a photosynthetic MFC that utilizes a purple non-sulfur bacterium. The MFC prototype they constructed was found to function over a range of environmental conditions, suggesting its potential use in industrial models.
Read More...Developing a Portable, Reusable, and Inexpensive Magnesium-Air Fuel Cell
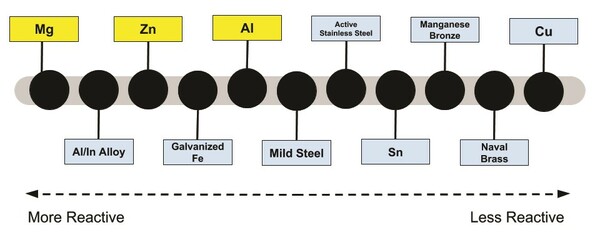
One of the greatest challenges we face today is the sustainable production, storage, and distribution of electrical power. One emerging technology with great promise in this area is that of metal-air fuel cells—a long-term and reusable electricity storage system made from a reactive metal anode and a saline solution. In this study the authors tested several different types of metal to determine which was the most suitable for this application. They found that a fuel cell with a magnesium anode was superior to fuel cells made from aluminum or zinc, producing a voltage and current sufficient for real-world applications such as charging a mobile phone.
Read More...Analysis of electrodialysis as a method of producing potable water
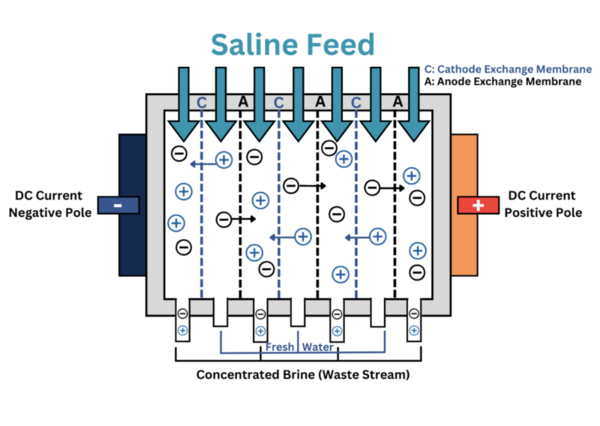
Here, seeking a way to convert the vast quantity of seawater to drinking water, the authors investigated the purification of seawater to drinking water through electrodialysis. Using total dissolved solids (TDS) as their measure, they found that electrodialysis was able to produce deionized water with TDS values under the acceptable range for consumable water.
Read More...From Waste to Wealth: Making Millivolts from Microbes!

In this study, the authors report their successful efforts to increase voltage production in a Microbial Fuel Cell (MFC), which is a system in which microorganisms produce electricity while performing their normal metabolism.
Read More...Cathodal Galvanotaxis: The Effect of Voltage on the distribution of Tetrahymena pyriformis
.png)
The surface of the unicellular eukaryote, Tetrahymena pyriformis, is covered with thousands of hair-like cilia. These cilia are very similar to cilia of the human olfactory and respiratory tracts making them model organisms for studying cilia function and pathology. The authors of this study investigated the effect of voltage on T. pyriformis galvanotaxis, the movement towards an electrical stimulus. They observed galvanotaxis towards the cathode at voltages over 4V which plateau, indicating opening of voltage gated-ion channels to trigger movement.
Read More...The Effect of Cobalt Biomineralization on Power Density in a Microbial Fuel Cell
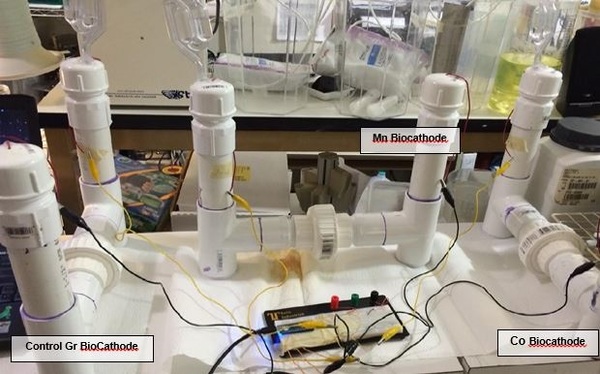
A microbial fuel cell is a system to produce electric current using biochemical products from bacteria. In this project authors operated a microbial fuel cell in which glucose was oxidized by Shewanella oneidensis in the anodic compartment. We compared the power output from biomineralized manganese or cobalt oxides, reduced by Leptothrix cholodnii in the cathodic compartment.
Read More...Discovery of the Heart in Mathematics: Modeling the Chaotic Behaviors of Quantized Periods in the Mandelbrot Set
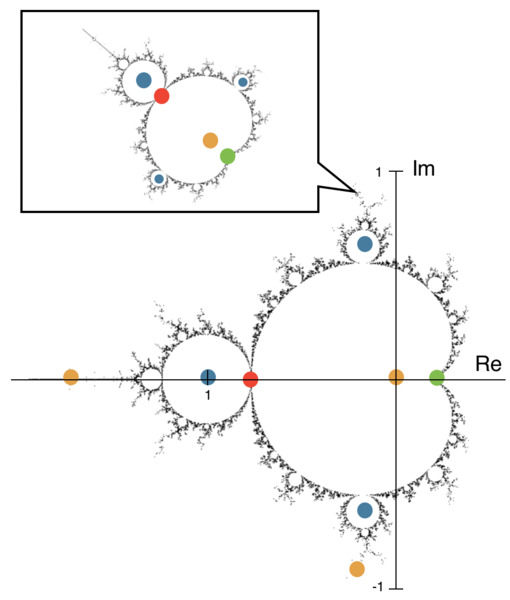
This study aimed to predict and explain chaotic behavior in the Mandelbrot Set, one of the world’s most popular models of fractals and exhibitors of Chaos Theory. The authors hypothesized that repeatedly iterating the Mandelbrot Set’s characteristic function would give rise to a more intricate layout of the fractal and elliptical models that predict and highlight “hotspots” of chaos through their overlaps. The positive and negative results from this study may provide a new perspective on fractals and their chaotic nature, helping to solve problems involving chaotic phenomena.
Read More...