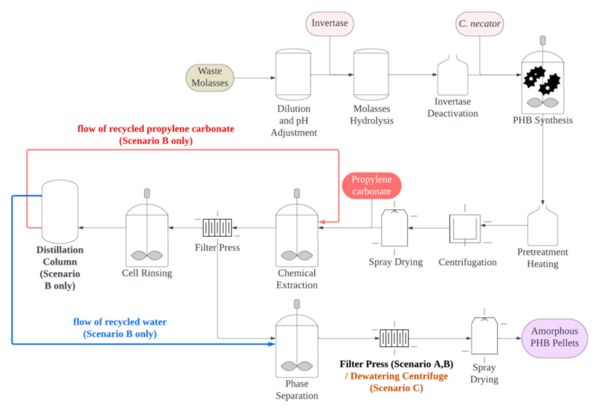
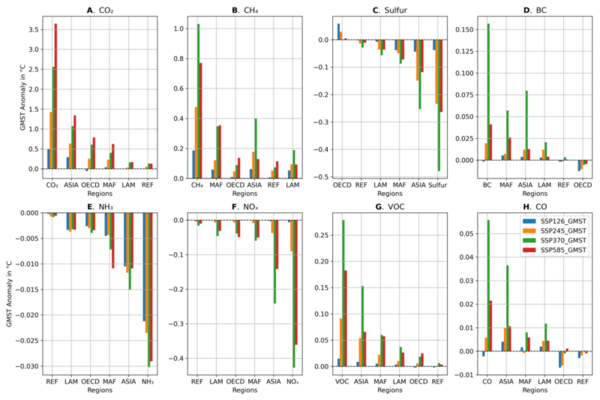
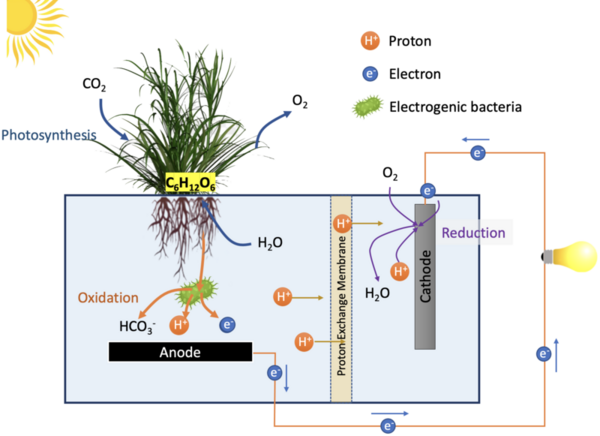
Every year, more than 30% of food products go to waste. This is approximately 1.3 billion tons of food, which is equivalent to 1.3 trillion U.S. dollars. While conventional solid waste treatments and fertilization of food waste are common, citrus fruit peels require secondary applications and advanced disposal management due to their low pH values and high antimicrobial characteristics. Since citrus fruits are well-known sources of vitamin C and antioxidants, we hypothesized that their peels also contain high amounts of vitamin C and antioxidants. In our study, five common citrus peels including grapefruit, lemon, lime, orange, and tangerine, were used to determine the amounts of vitamin C and total soluble antioxidants.
Read More...