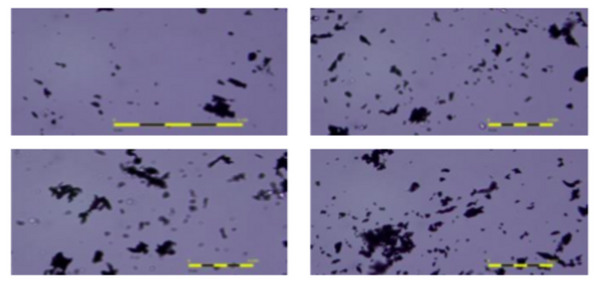
In this article, the authors propose an effective, environmentally-friendly method of producing conductive ink using expired waste oil, polystyrene, and graphene.
Read More...Environmentally-friendly graphene conductive ink using graphene powder, polystyrene, and waste oil

In this article, the authors propose an effective, environmentally-friendly method of producing conductive ink using expired waste oil, polystyrene, and graphene.
Read More...Optimal pH for indirect electrochemical oxidation of isopropyl alcohol with Ru-Ti anode and NaCl electrolyte

In this study, the authors determine optimal pH levels for maximizing isopropanol degradation in water. This has important applications for cleaning up polluted wastewater in the environment.
Read More...Alkaloids Detection in Commonly Found Medicinal Plants with Marquis Reagent

This study investigates the presence of alkaloids in a variety of medicinal plants using the Marquis reagent. They reveal some surprising results and how useful the Marquis reagent is.
Read More...Analysis of reduction potentials to determine the most efficient metals for electrochemical cell alternatives
In this study, the authors investigate what metals make the most efficient electrochemical cells, which are batteries that use the difference in electrical potential to generate electricity. Calculations predicted that a cell made of iron and magnesium would have the highest efficiency. Construction of an electrochemical cell of iron and magnesium produced voltages close to the theoretical voltage predicted. These findings are important as work continues towards making batteries with the highest storage efficiency possible.
Read More...The Cohesiveness of the Oscillating Belousov-Zhabotinsky Reaction

In this study the author undertakes a careful characterization of a special type of chemical reaction, called an oscillating Belousov-Zhabotinsky (or B-Z) reaction, which has a number of existing applications in biomedical engineering as well as the potential to be useful in future developments in other fields of science and engineering. Specifically, she uses experimental measurements in combination with computational analysis to investigate whether the reaction is cohesive – that is, whether the oscillations between chemical states will remain consistent or change over time as the reaction progresses. Her results indicate that the reaction is not cohesive, providing an important foundation for the development of future technologies using B-Z reactions.
Read More...The Effect of Concentration on the Pressure of a Sodium Chloride Solution Inside Dialysis Tubing
In this study, the authors investigate the effects of sodium levels on blood pressure, one of the most common medical problems worldwide. They used a simulated blood vessel constructed from dialysis tubing to carefully analyze pressure changes resulting from various levels of sodium in the external solution. They found that when the sodium concentration in the simulated blood vessel was higher than the external fluid, internal pressure increased, while the reverse was true when the sodium concentration was lower than in the surrounding environment. These results highlight the potential for sodium concentration to have a significant effect on blood pressure in humans by affecting the rate of osmosis across the boundaries of actual blood vessels.
Read More...Fabrication of CuSbS2 Solar Cells by Sulfurization of Thermally Evaporated Metal Stacks
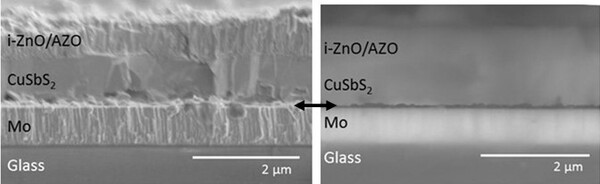
In this article, the authors created CuSbS2 solar cells. They discovered that the cells' efficiency was affected by the formation of MoS2. By incorporating a layer of single-walled carbon nanotubes, however, they were able to prevent MoS2 formation and increase the device's efficiency.
Read More...High-performance liquid chromatography insight in pH-dependent hydrolysis of andrographolide acetonide
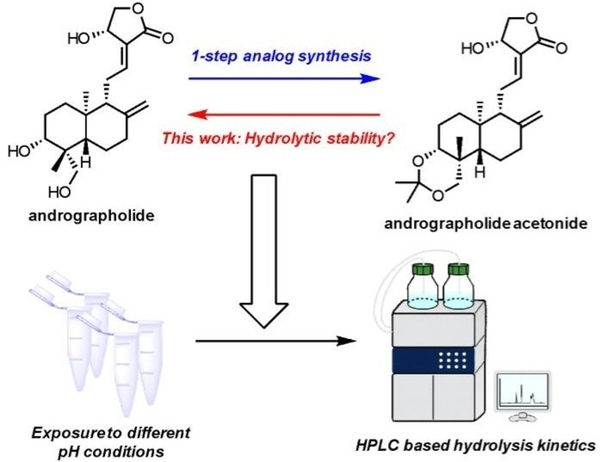
Andrographolide, a natural compound with anti-inflammatory, antidepressant, and anti-cancer properties, can be chemically modified by adding an acetonide group to form andrographolide acetonide, which is more potent and acts as a pH-dependent prodrug. Researchers investigated the hydrolysis of this acetonide group under mildly acidic conditions.
Read More...Mechanistic deconvolution of autoreduction in tetrazolium-based cell viability assays
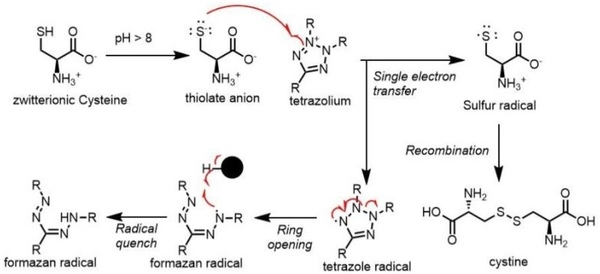
Optical reporters like tetrazolium dyes, exemplified by 5-diphenyl tetrazolium bromide (MTT), are effective tools for quantifying cellular responses under experimental conditions. These dyes assess cell viability by producing brightly-colored formazan dyes when reduced inside active cells. However, certain small molecules, including reducing agents like ascorbic acid, cysteine, and glutathione (GSH), can interfere with MTT assays, potentially compromising accuracy.
Read More...Hammett linear free-energy relationships in the biocatalytic hydrolysis of para-substituted nitrophenyl benzoate esters
As the world moves towards more eco-friendly methods for chemical synthesis, there's a strong interest in employing enzymes in chemical synthetic processes. Here, the authors explore how the activity of enzymes such as trypsin, lipase and nattokinase is affected by the electronic effects of the substrate they are acting on.
Read More...