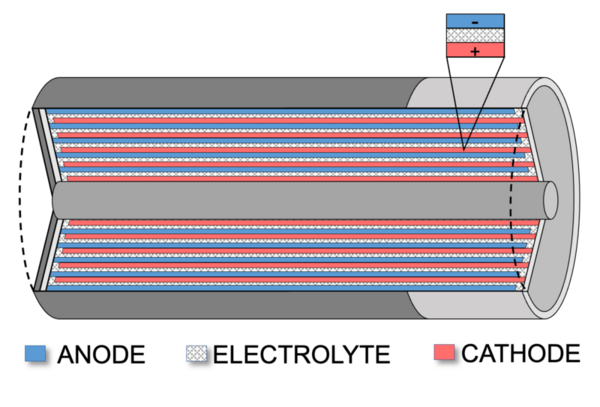
Rechargeable batteries are playing an increasingly prominent role in our lives due to the ongoing transition from fossil energy sources to green energy. The purpose of this study was to investigate variables that impact the effectiveness of rechargeable batteries. Alkaline (non-rechargeable) and rechargeable batteries share common features that are critical for the operation of a battery. The positive and negative electrodes, also known as the cathode and anode, are where the energy of the battery is stored. The electrolyte is what facilitates the transfer of cations and anions in a battery to generate electricity. Due to the importance of these components, we felt that a systematic investigation examining the surface area of the cathode and anode as well the impact of electrolytes with different properties on battery performance was justified. Utilizing a copper cathode and aluminum anode coupled with a water in salt electrolyte, a model rechargeable battery system was developed to test two hypotheses: a) increasing the contact area between the electrodes and electrolyte would improve battery capacity, and b) more soluble salt-based electrolytes would improve battery capacity. After soaking in an electrolyte solution, the battery was charged and the capacity, starting voltage, and ending voltage of each battery were measured. The results of this study supported our hypothesis that larger anode/cathodes surface areas and more ionic electrolytes such as sodium chloride, potassium chloride and potassium sulfate resulted in superior battery capacity. Incorporating these findings can help maximize the efficiency of commercial rechargeable batteries.
Read More...

.PNG)