
In this study, the authors determine optimal pH levels for maximizing isopropanol degradation in water. This has important applications for cleaning up polluted wastewater in the environment.
Read More...Optimal pH for indirect electrochemical oxidation of isopropyl alcohol with Ru-Ti anode and NaCl electrolyte

In this study, the authors determine optimal pH levels for maximizing isopropanol degradation in water. This has important applications for cleaning up polluted wastewater in the environment.
Read More...Low environmental pH inhibits phagosome formation and motility of Tetrahymena pyriformis
.jpg)
In this study, the authors look into some of the implications of rising carbon dioxide levels by studying the effects of acidic pH on the ability of T. pyriformis to feed by quantifying phagosome formation and motility.
Read More...Effect of pH Change on Exoskeletons of Selected Saltwater Organisms Which Rely on Calcium Fixation
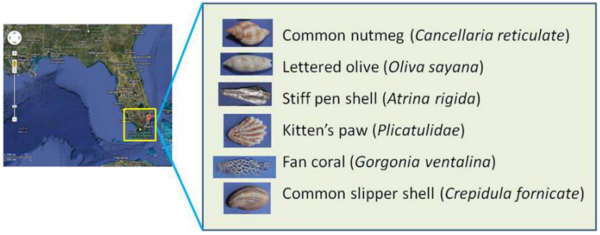
Rising atmospheric carbon dioxide levels are projected to lead to a 0.3- 0.4 unit decrease in ocean surface pH levels over the next century. In this study, the authors investigate the effect of pH change on the mass of calcified exoskeletons of common aquatic organisms found in South Florida coastal waters.
Read More...Pediatric probiotic culture survival study in acidic pH using an in vitro model
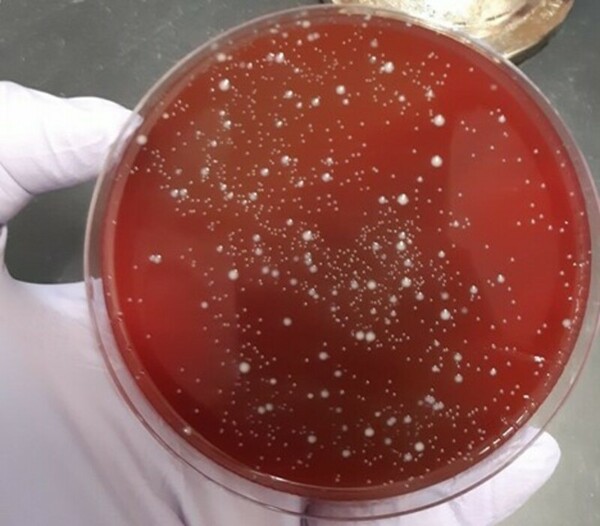
In this study, the authors investigate the effects of acidity on the survival of commercial probiotic Lovebug bacterial strains.
Read More...Developing a neural network to model the mechanical properties of 13-8 PH stainless steel alloy

We systematically evaluated the effects of raw material composition, heat treatment, and mechanical properties on 13-8PH stainless steel alloy. The results of the neural network models were in agreement with experimental results and aided in the evaluation of the effects of aging temperature on double shear strength. The data suggests that this model can be used to determine the appropriate 13-8PH alloy aging temperature needed to achieve the desired mechanical properties, eliminating the need for many costly trials and errors through re-heat treatments.
Read More...Effects of Ocean Acidification on the Photosynthetic Ability of Chaetoceros gracilis in the Monterey Bay

In this article, Harvell and Nicholson hypothesized that increased ocean acidity would decrease the photosynthetic ability of Chaetoceros gracilis, a diatom prolific in Monterey Bay, because of the usually corrosive effects of carbonic acid on both seashells and cells’ internal structures. They altered pH of algae environments and measured the photosynthetic ability of diatoms over four days by spectrophotometer. Overall, their findings indicate that C. gracilis may become more abundant in Monterey Bay as the pH of the ocean continues to drop, potentially contributing to harmful algal blooms.
Read More...Reduce the harm of acid rain to plants by producing nitrogen fertilizer through neutralization

The phenomenon of dying trees and plants in areas affected by acid rain has become increasingly problematic in recent times. Is there any method to efficiently utilize the rainwater and reduce the harmfulness of acid rain or make it beneficial to plants? This study aimed to investigate the potential of neutralizing acid rainwater infiltrating the soil to increase soil pH, produce beneficial salts for plants, and support better plant growth. To test this hypothesis, precipitation samples were collected from six states in the U.S. in 2022, and the pH of the acid rain was measured to obtain a representative pH value for the country. Experiments were then conducted to simulate the neutralization of acid rain and the subsequent change in soil pH levels. To evaluate the effectiveness and feasibility of this method, cat grass was planted in pots of soil soaked with solutions mimicking acid rain, with control and experimental groups receiving neutralizing agents (ammonium hydroxide) or not. Plant growth was measured by analyzing the height of the plants. Results demonstrated that neutralizing agents were effective in improving soil pH levels and that the resulting salts produced were beneficial to the growth of the grass. The findings suggest that this method could be applied on a larger agricultural scale to reduce the harmful effects of acid rain and increase agricultural efficiency.
Read More...Detergent pollutants decrease nutrient availability in soil
Household detergents have surfactants that can potentially harm the soil and broader ecosystems. In this study, the authors investigate whether eco-friendly and less-eco-friendly detergents affect soil pH, phosphorus, nitrogen, and potassium levels.
Read More...Effects of airport runoff pollution on water quality in bay area sites near San Francisco and Oakland airports
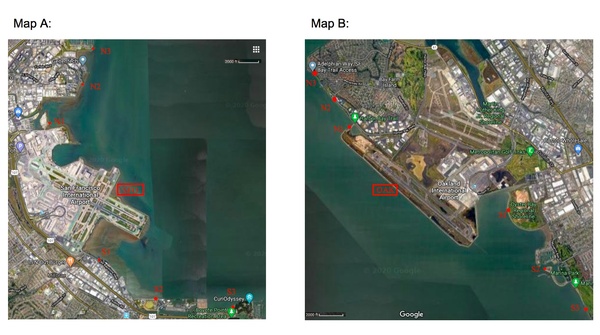
In this study, the authors sample water at different points closer and closer to two different airports to determine if these airports may be contributing to water pollution, specifically by measuring metals, nitrates, and pH.
Read More...Fluorescein or Green Fluorescent Protein: Is It Possible to Create a Sensor for Dehydration?

Currently there is no early dehydration detection system using temperature and pH as indicators. A sensor could alert the wearer and others of low hydration levels, which would normally be difficult to catch prior to more serious complications resulting from dehydration. In this study, a protein fluorophore, green fluorescent protein (GFP), and a chemical fluorophore, fluorescein, were tested for a change in fluorescence in response to increased temperature or decreased pH. Reversing the pH change did not restore GFP fluorescence, but that of fluorescein was re-established. This finding suggests that fluorescein could be used as a reusable sensor for a dehydration-related pH change.
Read More...