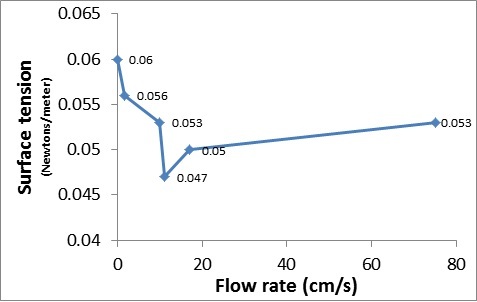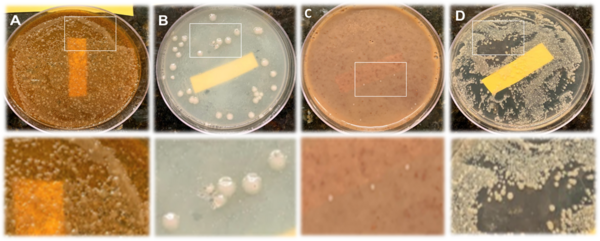
Bacteria cause tooth decay, plaque, bad breath, and other diseases. Despite being cleaned with water and toothpaste, oral bacteria live on our toothbrushes. Bacterial growth has been shown to be inhibited by different toothpastes and common household spices. This study tested how different toothpastes and common household spices, including cinnamon, cumin, nutmeg, and ground white pepper, can inhibit bacteria from growing on toothbrushes
Read More...