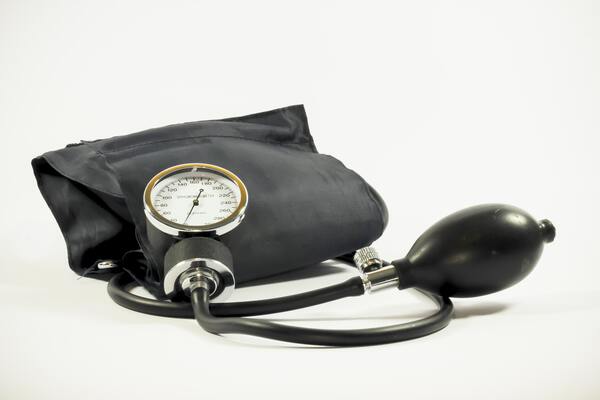
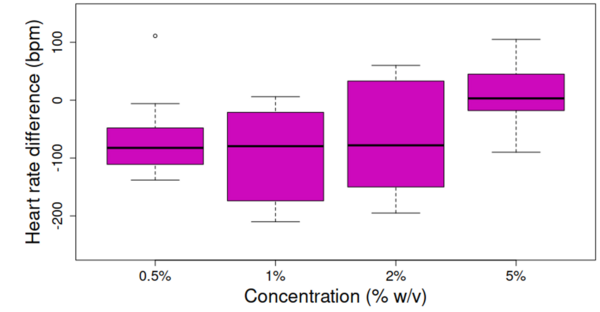
In this study, the authors investigate the effects of sodium levels on blood pressure, one of the most common medical problems worldwide. They used a simulated blood vessel constructed from dialysis tubing to carefully analyze pressure changes resulting from various levels of sodium in the external solution. They found that when the sodium concentration in the simulated blood vessel was higher than the external fluid, internal pressure increased, while the reverse was true when the sodium concentration was lower than in the surrounding environment. These results highlight the potential for sodium concentration to have a significant effect on blood pressure in humans by affecting the rate of osmosis across the boundaries of actual blood vessels.
Read More...
.jpg)