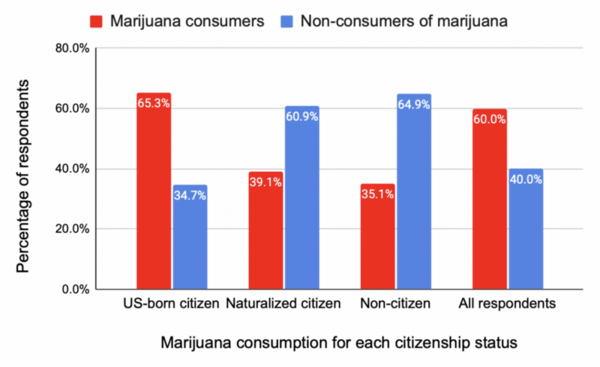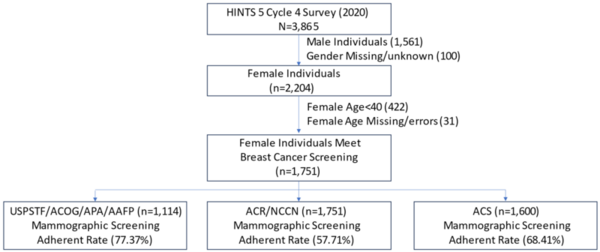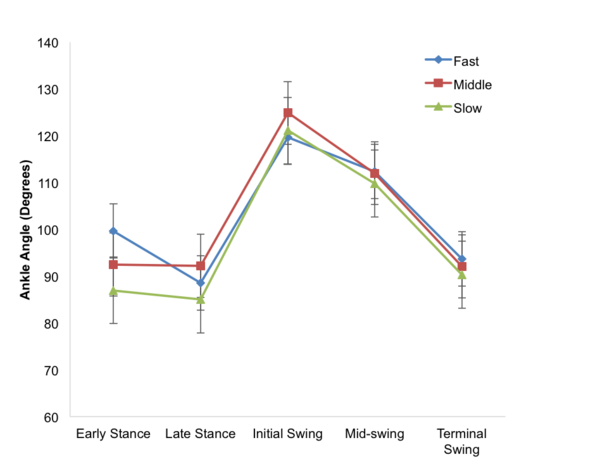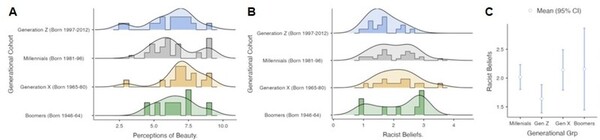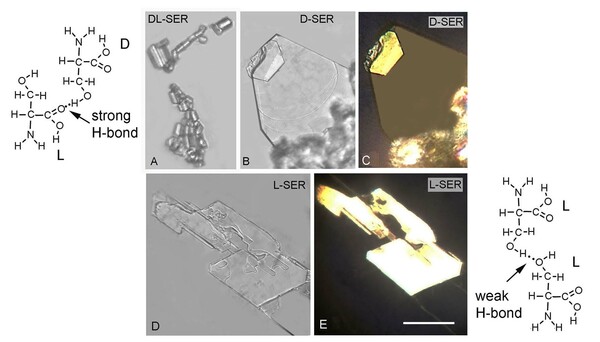
Seeking to develop a better understanding of the chemical and physical properties of amino acids that compose proteins, here the authors investigated the unusual relative insolubility of racemic mixtures of D- and L-serine compared to the solubility of pure D- or L-serine. The authors used a combination of microscopy and temperature measurements alongside previous X-ray diffraction studies to conclude that racemic DL-serine crystals consist of comparatively stronger hydrogen bond interactions compared to crystals of pure enantiomers. These stronger interactions were found to result in the unique release of heat during the crystallization of racemic mixtures.
Read More...