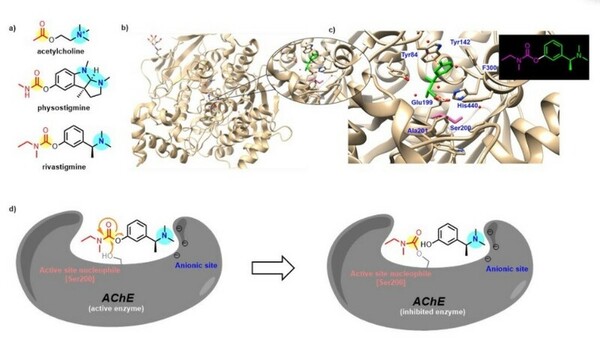
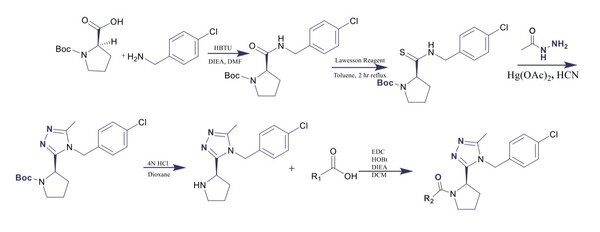
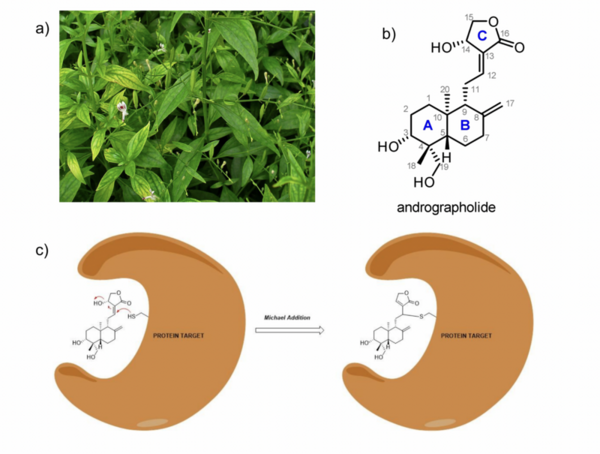
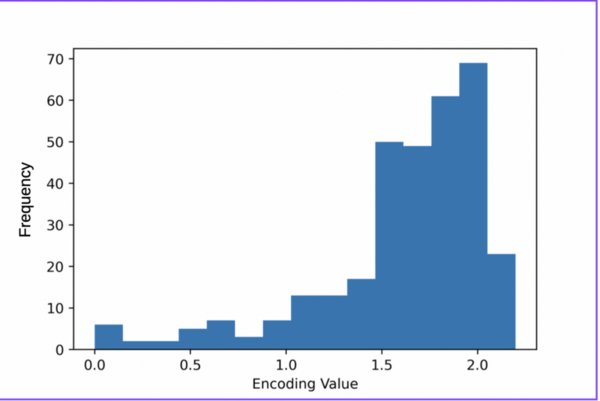
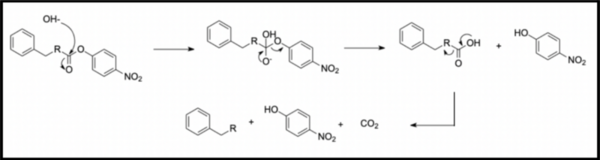
Irrespective of the final application of a molecule, synthetic accessibility is the rate-determining step in discovering and developing novel entities. However, synthetic complexity is challenging to quantify as a single metric, since it is a composite of several measurable metrics, some of which include cost, safety, and availability. Moreover, defining a single synthetic accessibility metric for both natural products and non-natural products poses yet another challenge given the structural distinctions between these two classes of compounds. Here, we propose a model for synthetic accessibility of all chemical compounds, inspired by the Central Limit Theorem, and devise a novel synthetic accessibility metric assessing the overall feasibility of making chemical compounds that has been fitted to a Gaussian distribution.
Read More...
.jpeg)