High-performance liquid chromatography insight in pH-dependent hydrolysis of andrographolide acetonide
(1) Irvington High School, (2) Notre Dame High School, (3) Mission San Jose High School, (4) Adrian C. Wilcox High School, (5) Leigh High School, (6) American High School, (7) Valley Christian High School, (8) Fremont High School, (9) Washington High School, (10) St. Francis High School, (11) Dougherty Valley High School, (12) Department of Chemistry, Biochemistry & Physics, Aspiring Scholars Directed Research Program
https://doi.org/10.59720/22-281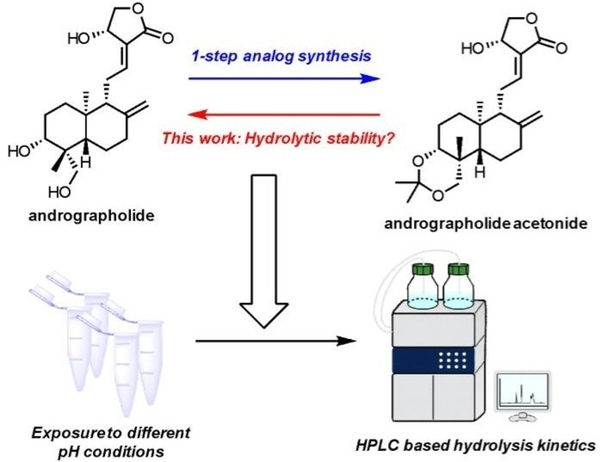
Andrographolide is a natural labdane diterpenoid that functions as an inhibitor of Nuclear factor Kappa B (Nf-kB), and has demonstrated anti-inflammatory, anti-depressant, and anti-cancerous properties. In a one-step chemical synthesis, the 3,19-diol system of andrographolide can be protected with an acetonide group, yielding 3,19-isopropylidene andrographolide (“andrographolide acetonide”). Acetonide protecting groups are known to be unstable under mildly acidic conditions, and previous literature has reported that andrographolide acetonide is more potent than andrographolide. This led us to believe that 3,19-isopropylidene andrographolide exerts its biological activity as a pH-dependent prodrug of andrographolide. Using high performance liquid chromatography to create time courses that quantify the rate at which the acetonide group cleaves off of andrographolide acetonide in various mildly acidic pH buffers, we determined that the rate of hydrolysis was a first order reaction. In order to ascertain which mechanism of hydrolysis is more probable, we utilized density functional theory (DFT) to calculate the absolute free energies of the two initial rate-determining protonated intermediates, coming to the conclusion that C19 is preferentially protonated over C3 by 4.33 kcal/mol. Understanding the nature of andrographolide acetonide hydrolysis under different conditions lays the groundwork for improving both the stability and potency of andrographolide analogs via chemical synthesis.
This article has been tagged with: