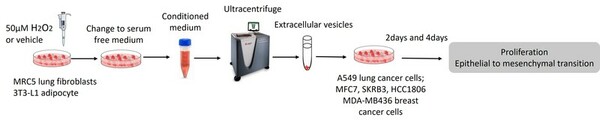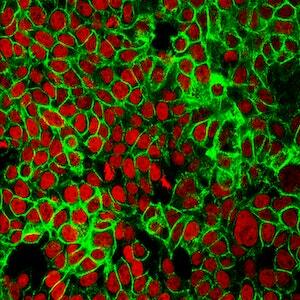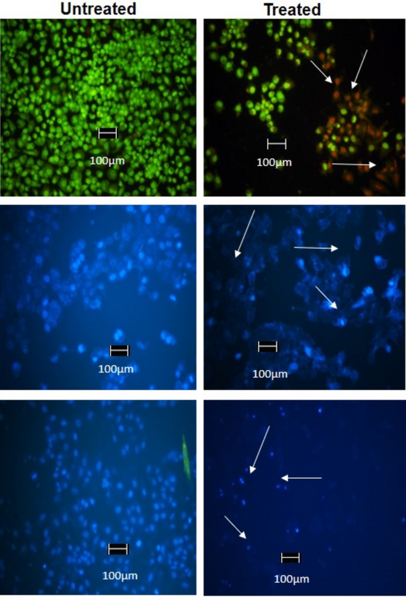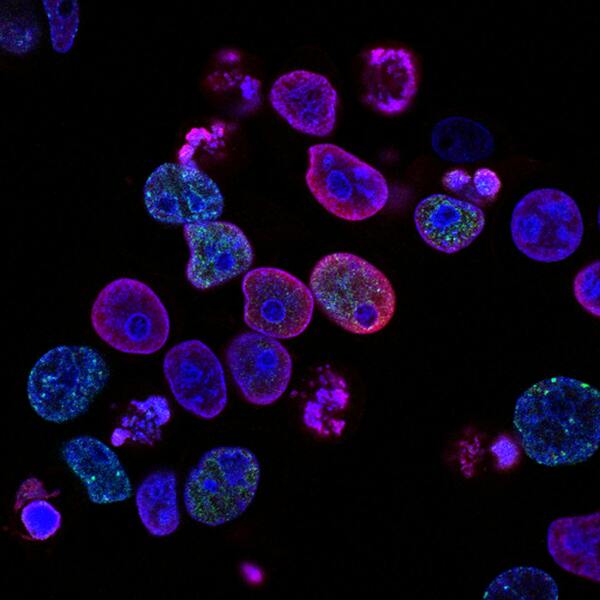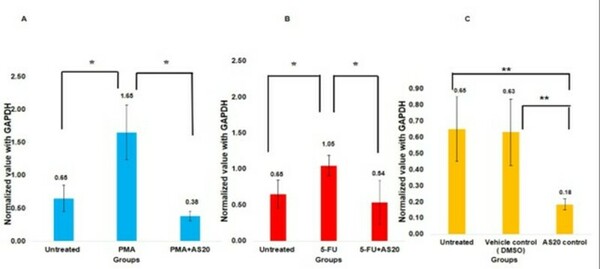
Mesenchymal stem cells(MSCs) play a role in tumor formation by differentiating into cancer associated fibroblasts (CAFs) which enable metastasis of tumors. The process of conversion of MSCs into CAFs is not clear. In this study, authors tested the hypothesis that cancers cells secrete soluble factors that induce differentiation by culturing bone marrow mesenchymal stem cells in media conditioned by a breast cancer cell line.
Read More...
.jpg)