Taft linear free-energy relationships in the biocatalytic hydrolysis of sterically hindered nitrophenyl ester substrates
(1) Leigh High School, San Jose, CA, (2) Department of Chemistry, Biochemistry, & Physical Science, Aspiring Scholars Directed Research Program, Fremont, California
https://doi.org/10.59720/21-130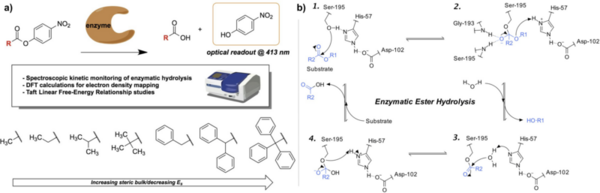
Linear free-energy relationships (LFERs) have been commonly used to uncover reaction mechanisms in organic chemistry by correlating trends in reactivity to reactant properties. However, the applications of LFERs have largely been limited to traditional organic synthesis and have much less frequently been applied toward enzyme-catalyzed reactions. In this study, we used the Taft LFER, which correlates reaction rates with steric properties of reactants, to study kinetic trends in the enzymatic hydrolysis of sterically hindered substrates. We synthesized 4-nitrophenyl ester compounds with substituents of varying degrees of steric hindrance, and then subjected these compounds to hydrolysis by the enzymes lipase, trypsin, and nattokinase. Kinetic data was obtained by using a spectrophotometer to monitor the formation of 4-nitrophenol, a bright yellow product of the ester hydrolysis with an optical readout at 413 nm. Contrary to initial hypotheses, Taft plots did not exhibit linear relationships and further analysis yielded mechanistic insight into the nature of the Taft steric parameter, the relative sensitivity of each enzyme to steric effects, and potential enzyme-substrate binding interactions. This analysis was paralleled with computational calculations to determine local charge density of the reaction center, which supported that the unexpected trends were largely a function of the aforementioned factors rather than electronic effects. Ultimately, we demonstrate the unconventional application of the Taft LFER toward biocatalytic transformations and open avenues toward the broader use of biocatalysts in synthetic organic chemistry.
This article has been tagged with: