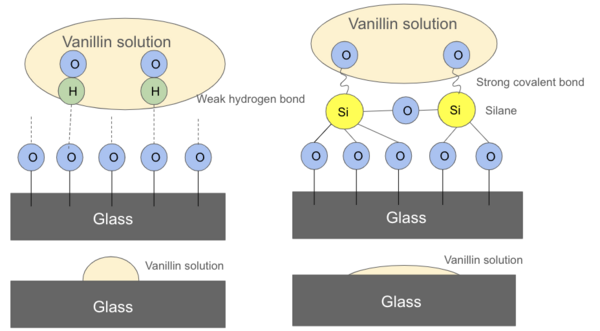
In this study, the authors investigate the crystallization kinetics of vanillin thin films from a solution or a melt, as well as how a silane coating on the glass surface affects these properties.
Read More...Crystallization kinetics of vanillin thin films

In this study, the authors investigate the crystallization kinetics of vanillin thin films from a solution or a melt, as well as how a silane coating on the glass surface affects these properties.
Read More...Deuterated solvent effects in the kinetics and thermodynamics of keto-enol tautomerization of ETFAA
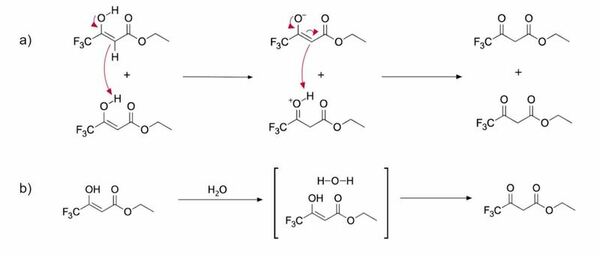
In this study, the authors determined whether tautomerization dynamics in protic and aprotic solvents displayed differences in reaction rates and in the proportion of the keto and enol tautomers present.
Read More...Reactivity-informed design, synthesis, and Michael addition kinetics of C-ring andrographolide analogs
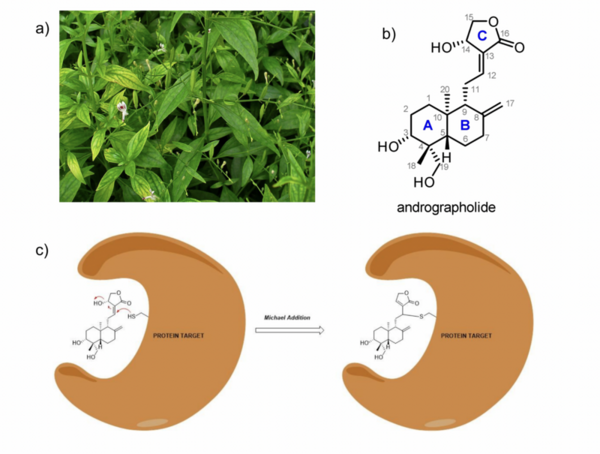
Here, based on the identification of androgapholide as a potential therapeutic treatment against cancer, Alzheimer's disease, diabetes, and multiple sclerosis, due to its ability to inhibit a signaling pathway in immune system function, the authors sought ways to optimize the natural product human systems by manipulating its chemical structure. Through the semisynthesis of a natural product along with computational studies, the authors developed an understanding of the kinetic mechanisms of andrographolide and semisynthetic analogs in the context of Michael additions.
Read More...Using the COmplex PAthway SImulator, Stage Analysis, and Chemical Kinetics to Develop a Novel Solution to Lower Tau Concentrations in Alzheimer’s Disease
In this study, the authors ask whether a Tau immunotherapy treatment, Hsp70 protein treatment, or dual treatment approach of both the Tau imunotherapy treatment and Hsp70 protein treatment leads to a greater reduction in Tau protein concentration in Alzheimer's disease. Overall, they conclude that the effectiveness of the treatment ultimately relies on the stage of Alzheimer’s.
Read More...Assessing CDK5 as a Nanomotor for Chemotactic Drug Delivery
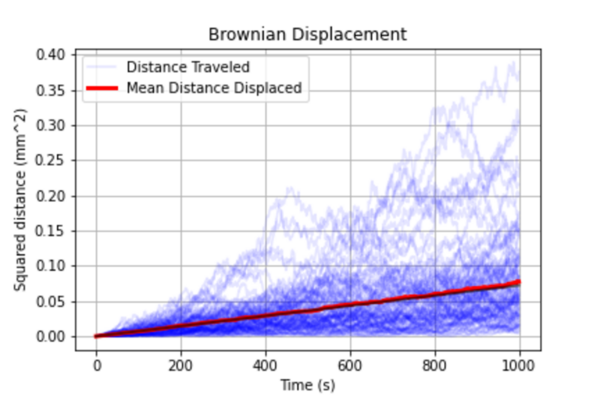
Enzyme chemotaxis is a thermodynamic phenomenon in which enzymes move along a substrate concentration gradient towards regions with higher substrate concentrations and can be used to steer nanovehicles towards targets along natural substrate concentrations. In patients with Alzheimer’s disease, a gradient of tau protein forms in the bloodstream. Tau protein is a substrate of the enzyme CDK5, which catalyzes the phosphorylation of tau protein and can travel using chemotaxis along tau protein gradients to increasing concentrations of tau and amyloid-beta proteins. The authors hypothesized that CDK5 would be able to overcome these barriers of Brownian motion and developed a quantitative model using Michaelis-Menten kinetics to define the necessary parameters to confirm and characterize CDK5’s chemotactic behavior to establish its utility in drug delivery and other applications.
Read More...The Effect of Cobalt Biomineralization on Power Density in a Microbial Fuel Cell
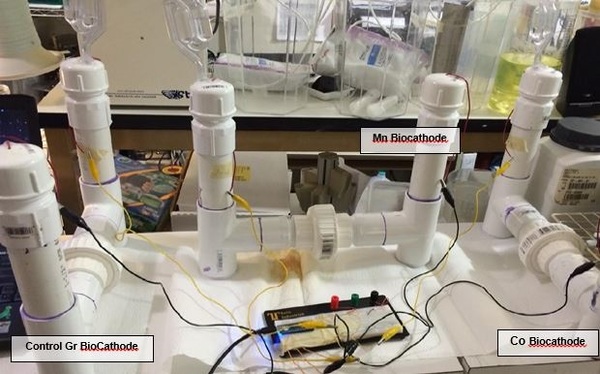
A microbial fuel cell is a system to produce electric current using biochemical products from bacteria. In this project authors operated a microbial fuel cell in which glucose was oxidized by Shewanella oneidensis in the anodic compartment. We compared the power output from biomineralized manganese or cobalt oxides, reduced by Leptothrix cholodnii in the cathodic compartment.
Read More...In vitro dissolution and in vivo response of pseudoephedrine dosage forms
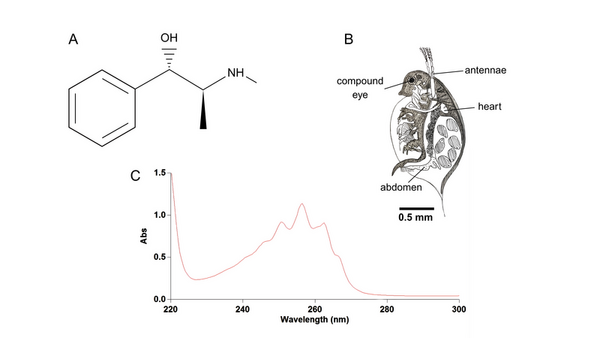
The authors looked at how pharmacokinetics changed depending on the use of an in vitro or an in vivo model.
Read More...Exploring the possibilities for reactions between SiW and alkaline solutions to be renewable energy sources
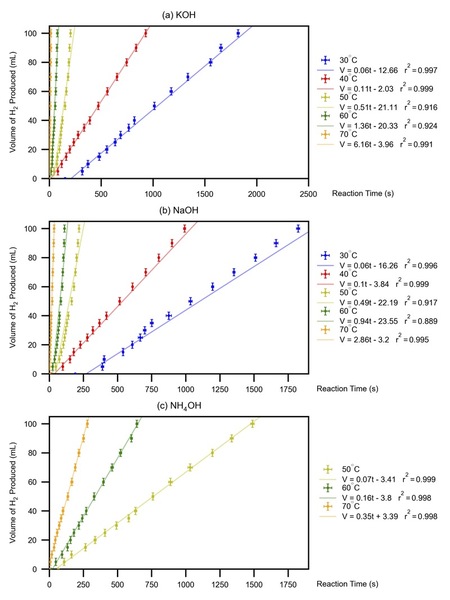
The authors looked at hydrogen gas production and how reaction temperature, concentration and alkaline solution used impacted the overall reaction with silicon. They found that all alkaline solutions tested would be viable options for using silicon waste to produce hydrogen gas to be used a renewable energy source.
Read More...Spectroscopic Kinetic Monitoring and Molecular Dynamics Simulations of Biocatalytic Ester Hydrolysis in Non-Aqueous Solvent
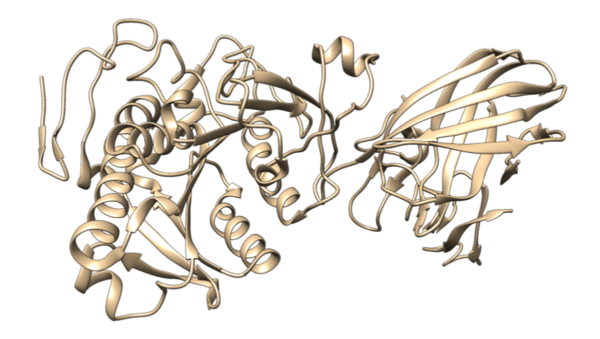
Lipases are a common class of enzymes that catalyze the breakdown of lipids. Here the authors characterize the the activity of pancreatic lipase in different organic solvents using a choloremetric assay, as well as using molecular dynamic simulations. They report that the activity of pancreatic lipase in 5% methanol is more than 25% higher than in water, despite enzyme stability being comparable in both solvents. This suggests that, for industrial applications, using pancreatic lipase in 5% methanol solution might increase yield, compared to just water.
Read More...Kinetic Monitoring and Fourier-Transform Infrared (FTIR) Spectroscopy of the Green Oxidation of (-)-Menthol to (-)-Menthone
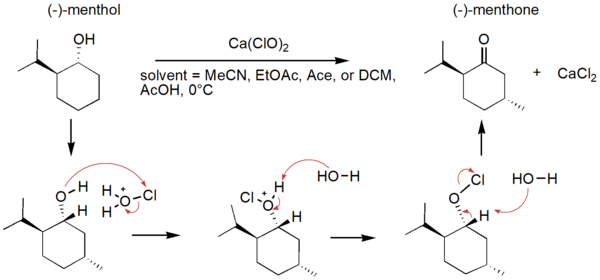
In an effort to reduce the production of hazardous substances, green chemistry aims to make chemical processes more sustainable. One way to do so is changing solvents in chemical reactions. Here, authors assessed different “green” solvents on the oxidation of (-)-menthol to (-)-menthone using Fourier-transform infrared (FTIR) spectroscopy, optimizing the solvent system for this reaction.
Read More...