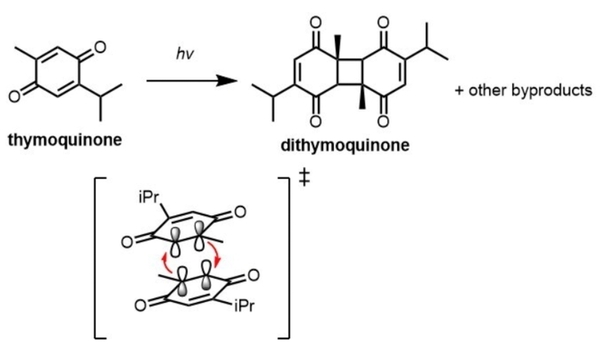
Thymoquinone is a compound of great therapeutic potential and scientific interest. However, its clinical administration and synthetic modifications are greatly limited by its instability in the presence of light. This study employed quantitative 1H nuclear magnetic resonance (NMR) spectroscopy to identify the effect of solvation on the degradation of thymoquinone under ultraviolet light (UV). It found that the rate of degradation is highly solvent dependent occurs maximally in chloroform.
Read More...