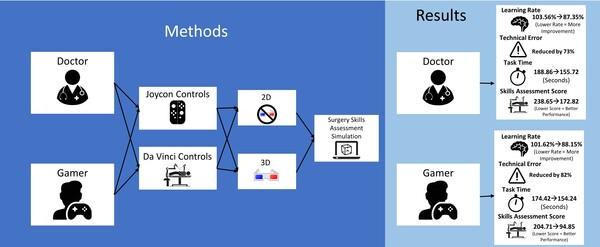
Cystic fibrosis is a genetic disease caused by mutations in the CFTR gene. In this paper, the authors attempt to identify variations in stretches of up to 8 nucleotides in the protein-coding portions of the CFTR gene that are associated with disease development. This would allow screening of newborns or even fetuses in utero to determine the likelihood they develop cystic fibrosis.
Read More...