The sweetened actualities of neural membrane proteins: A computational structural analysis
(1) Sacramento Country Day School, Sacramento, California, (2) Cosumnes River College, Sacramento, California
https://doi.org/10.59720/21-070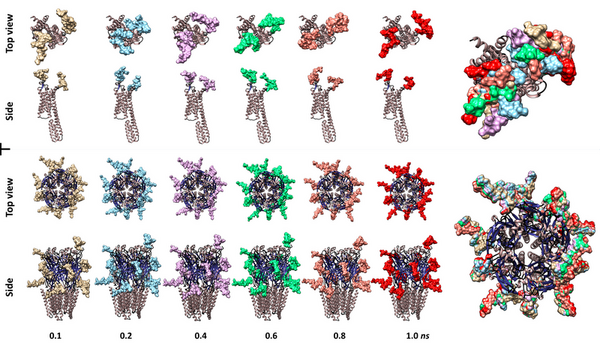
In nature, every cell is coated by an array of complex molecules known as glycans, which are also known as sugars. Their intricacy makes them difficult to analyze and as a consequence little is known about their function. However, in the last decade, compounding evidence points to glycans participating in critical roles in every cell type, including neurons. In fact, altered glycosylation has been correlated to neural disorders such as Alzheimer’s and Parkinson's disease. To learn more about these molecules, we investigated two neural membrane glycoproteins: the type-A γ-aminobutyric acid (GABAA) and the human 5-hydroxytryptamine 2B (5-HT2B) receptors by computational methods. We examined if the glycan structures could physically interfere with the receptor active site. To answer this question, we modeled different glycans on each protein. The model shows that the active sites are heavily glycosylated and all or several glycans are either directly on or bracketing the active site. Furthermore, molecular dynamics simulations demonstrated these molecules to be highly dynamic and in the case of 5-HT2B receptor, capable of covering the entire active site region. Therefore, validating our hypothesis that at least physically, glycans partakes with the active site functions. Future experimental work needs to be done to reveal how the absence or structure changes in these molecules relate to disease. This information can be used for future neurological disease treatments and possible cures.
This article has been tagged with: