Modular mimics of neuroactive alkaloids - design, synthesis, and cholinesterase inhibitory activity of rivastigmine analogs
(1) Amador Valley High School , (2) Los Altos High School Ringgold ID 290485 , (3) Dougherty Valley High School , (4) Milpitas High School Ringgold, (5) Dougherty Valley High School, (6) The Quarry Lane School , (7) American High School, (8) Palo Alto High School , (9) James Logan High School, (10) Carlmont High School, (11) Prospect High School, (12) Leigh High School, (13) California High School, (14) Aspiring Scholars Directed Research Program
https://doi.org/10.59720/22-070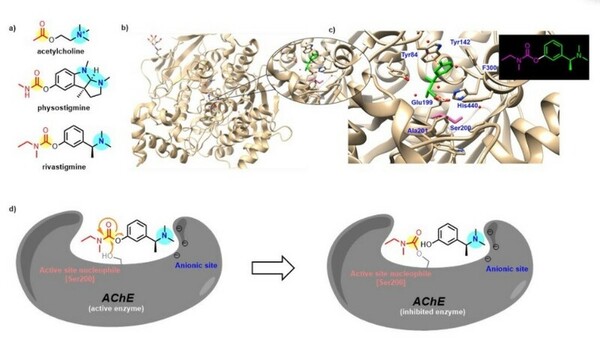
The treatment of neurological diseases has evolved to include neuroactive alkaloids isolated from naturally occurring phytochemical sources. While some of these compounds have gone on to clinical use themselves, others have inspired the development of synthetic analogs, which might possess greater potency or better pharmacological features than the natural product itself. One such naturally occurring alkaloid, physostigmine, which is found in the Calabar bean plant Physostigma venenosum, has been demonstrated to be a potent cholinesterase inhibitor. However, some of physostigmine's characteristics limit its therapeutic potential, prompting the development of rivastigmine, a similarly structured synthetic compound. The research in our group focused on the synthetic optimization of rivastigmine and its analogs, utilizing computer modeling and biological assays to determine the most favorable analog for inhibition of acetylcholinesterase (AChE), the enzyme that breaks down the neurotransmitter acetylcholine (ACh) to terminate neuronal transmission and signaling between synapses. Patients with Alzheimer’s Disease have lower levels of ACh, which has been associated with symptoms like memory impairment and confusion. The inhibition of AChE allows for ACh accumulation and continued signaling in parasympathetic nervous system. Through our studies, we determined that rivastigmine and its analogs were less effective at inhibiting AChE than physostigmine, and their biological activity is governed by sterics.
This article has been tagged with: