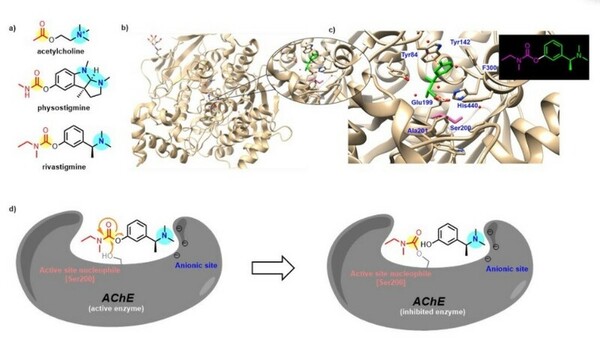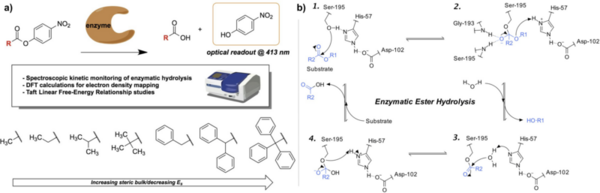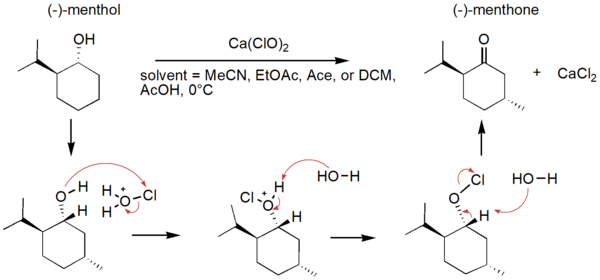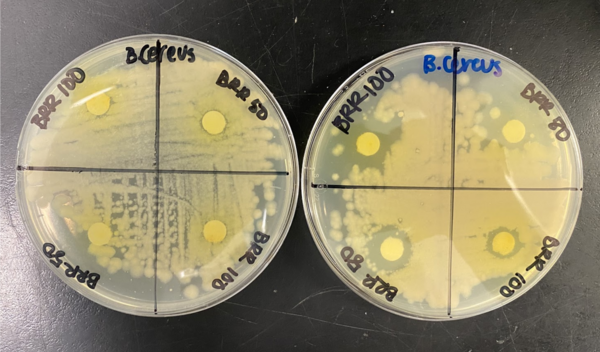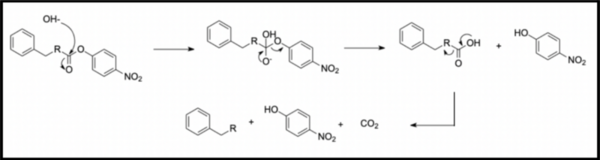
In organic synthesis, protecting groups are derivatives of reactive functionalities that play a key role in ensuring chemoselectivity of chemical transformations. To protect alcohols and amines, acid-labile tert-butyloxycarbonyl protecting groups are often employed but are avoided when the substrate is acid-sensitive. Thus, orthogonal base-labile protecting groups have been in demand to enable selective deprotection and to preserve the reactivity of acid-sensitive substrates. To meet this demand, we present 4-nitrophenyl carbonates and carbamates as orthogonal base-labile protecting group strategies.
Read More...