Strain selective in vitro and in silico structure activity relationship of tetracycline antibiotics
(1) Department of Chemistry, Biochemistry & Physics, Aspiring Scholars Directed Research Program; BASIS Independent Silicon Valley, (2) Department of Chemistry, Biochemistry & Physics, Aspiring Scholars Directed Research Program; Mission San Jose High School, (3) Department of Chemistry, Biochemistry & Physics, Aspiring Scholars Directed Research Program
https://doi.org/10.59720/22-241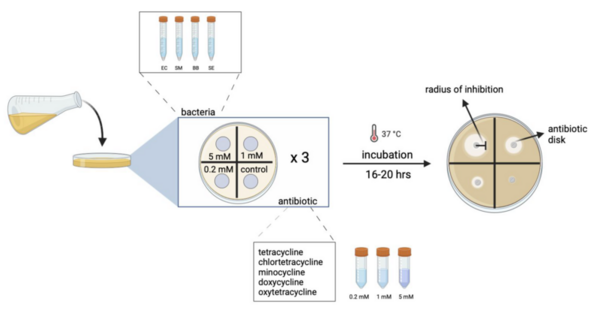
Since the discovery of penicillin G as an effective antibacterial agent by Alexander Flemming in 1928, the demand for new, potent antibiotics has continued to rise due to the development of antibiotic resistance. To combat this crisis, novel antibiotics have been created via optimizing existing structures or natural products found in nature. The tetracycline family of antibiotics is of particular interest due to their broad-spectrum effects. In this study we investigated the antibacterial effects of tetracycline and four of its analogs (chlortetracycline, doxycycline, minocycline, and oxytetracycline) against four species of bacteria. Tetracyclines exhibit bacteriostatic effects by binding to the highly conserved 30S ribosomal subunit of bacteria and interfering in protein synthesis as a result. We hypothesized that the in vitro antibacterial efficacy of each tetracycline would vary with each strain and have comparable binding affinities in silico. Through Kirby-Bauer disk diffusion assays, we found that the efficacy of those compounds within the tetracycline family was concentration- and strain-dependent. To further elucidate the structure-activity relationship exhibited in the in vitro assays, in silico virtual screens were conducted using density functional theory calculations and molecular docking. The computational results demonstrated that all compounds resulted in relatively similar binding affinities, posing a future potential in designing analogs to surpass currently available tetracycline antibiotics.
This article has been tagged with: