In vitro characterization of umilical cord-dervied MSC's supplemented with PLAY®:A potential FBS substitute
(1) Indus International School Bangalore, Bangalore, India, (2) iCrest, International Stem Cell Services Ltd., Bangalore, India
https://doi.org/10.59720/22-162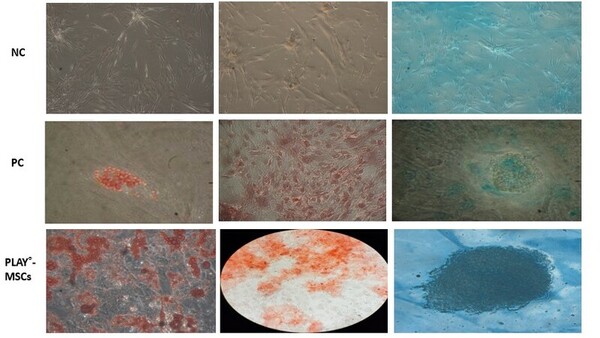
Recent interest in the wound healing and regenerative properties of mesenchymal stem cells (MSCs) has prompted further investigation to elucidate their regenerative properties. Characterized by their ability to differentiate into various cell lineages, such as osteocytes, chondrocytes, and adipocytes, alongside promoting local angiogenesis and chemotaxis at the wound site, MSCs have proven to reduce the stipulated healing time. The myriad sources they originate from, including bone marrow and umbilical cord, underscore their wide range of clinical applicability. Extending the time for MSC senescence by using alternative media in a manner that preserves their stem-cell-like nature may be a viable option of achieving optimum cell numbers required for clinical protocols. Furthermore, various culture conditions have varied effects on MSCs, therefore, standardizing MSC growth conditions may help to produce MSCs with verified potency, which could be then extrapolated to clinical conditions. The presence of potential contaminants within commonly used cell culture media such as fetal bovine serum (FBS) curbs the scope of MSC implementation in tissue regeneration. Our current study aimed to continue to investigate the effects of blood derivative PLAY® on trends in MSC culture across various experimental parameters in comparison to FBS. Our results demonstrated that PLAY® consistently outperformed FBS across all the experimental metrics studied.
This article has been tagged with: